Dynamic Cardiac Magnetic Resonance Fingerprinting During Vasoactive Breathing Maneuvers: First Results
- PMID: 37096671
- PMCID: PMC10133810
- DOI: 10.4250/jcvi.2022.0080
Dynamic Cardiac Magnetic Resonance Fingerprinting During Vasoactive Breathing Maneuvers: First Results
Abstract
Background: Cardiac magnetic resonance fingerprinting (cMRF) enables simultaneous mapping of myocardial T1 and T2 with very short acquisition times. Breathing maneuvers have been utilized as a vasoactive stress test to dynamically characterize myocardial tissue in vivo. We tested the feasibility of sequential, rapid cMRF acquisitions during breathing maneuvers to quantify myocardial T1 and T2 changes.
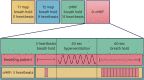
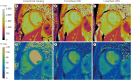
Methods: We measured T1 and T2 values using conventional T1 and T2-mapping techniques (modified look locker inversion [MOLLI] and T2-prepared balanced-steady state free precession), and a 15 heartbeat (15-hb) and rapid 5-hb cMRF sequence in a phantom and in 9 healthy volunteers. The cMRF5-hb sequence was also used to dynamically assess T1 and T2 changes over the course of a vasoactive combined breathing maneuver.
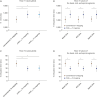
Results: In healthy volunteers, the mean myocardial T1 of the different mapping methodologies were: MOLLI 1,224 ± 81 ms, cMRF15-hb 1,359 ± 97 ms, and cMRF5-hb 1,357 ± 76 ms. The mean myocardial T2 measured with the conventional mapping technique was 41.7 ± 6.7 ms, while for cMRF15-hb 29.6 ± 5.8 ms and cMRF5-hb 30.5 ± 5.8 ms. T2 was reduced with vasoconstriction (post-hyperventilation compared to a baseline resting state) (30.15 ± 1.53 ms vs. 27.99 ± 2.07 ms, p = 0.02), while T1 did not change with hyperventilation. During the vasodilatory breath-hold, no significant change of myocardial T1 and T2 was observed.
Conclusions: cMRF5-hb enables simultaneous mapping of myocardial T1 and T2, and may be used to track dynamic changes of myocardial T1 and T2 during vasoactive combined breathing maneuvers.
Keywords: Breathing exercises; Magnetic resonance imaging; Multiparametric magnetic resonance imaging; Myocardium.
Copyright © 2023 Korean Society of Echocardiography.
Conflict of interest statement
Matthias G. Friedrich is listed as a holder of United States Patent No. 14/419,877: Inducing and measuring myocardial oxygenation changes as a marker for heart disease; United States Patent No. 15/483,712: Measuring oxygenation changes in tissue as a marker for vascular function; United States Patent No. 10,653,394: Measuring oxygenation changes in tissue as a marker for vascular function - continuation; and Canadian Patent CA2020/051776: Method and apparatus for determining biomarkers of vascular function utilizing bold CMR images.
Figures


Similar articles
-
Simultaneous Mapping of T1 and T2 Using Cardiac Magnetic Resonance Fingerprinting in a Cohort of Healthy Subjects at 1.5T.J Magn Reson Imaging. 2020 Oct;52(4):1044-1052. doi: 10.1002/jmri.27155. Epub 2020 Mar 28. J Magn Reson Imaging. 2020. PMID: 32222092 Free PMC article.
-
3D free-breathing cardiac magnetic resonance fingerprinting.NMR Biomed. 2020 Oct;33(10):e4370. doi: 10.1002/nbm.4370. Epub 2020 Jul 21. NMR Biomed. 2020. PMID: 32696590
-
Free-breathing simultaneous native myocardial T1, T2 and T1ρ mapping with Cartesian acquisition and dictionary matching.J Cardiovasc Magn Reson. 2023 Nov 9;25(1):63. doi: 10.1186/s12968-023-00973-6. J Cardiovasc Magn Reson. 2023. PMID: 37946191 Free PMC article.
-
Cardiac Magnetic Resonance Fingerprinting: Technical Overview and Initial Results.JACC Cardiovasc Imaging. 2018 Dec;11(12):1837-1853. doi: 10.1016/j.jcmg.2018.08.028. JACC Cardiovasc Imaging. 2018. PMID: 30522686 Free PMC article. Review.
-
Cardiac magnetic resonance fingerprinting: Trends in technical development and potential clinical applications.Prog Nucl Magn Reson Spectrosc. 2021 Feb;122:11-22. doi: 10.1016/j.pnmrs.2020.10.001. Epub 2020 Nov 6. Prog Nucl Magn Reson Spectrosc. 2021. PMID: 33632415 Free PMC article. Review.
Cited by
-
Dynamic Rapid Cardiac Magnetic Resonance Fingerprinting.J Cardiovasc Imaging. 2023 Apr;31(2):83-84. doi: 10.4250/jcvi.2022.0133. J Cardiovasc Imaging. 2023. PMID: 37096672 Free PMC article. No abstract available.
References
-
- Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magn Reson. 2017;19:75. - PMC - PubMed

