Ultrafast Laser Microlaryngeal Surgery for In Vivo Subepithelial Void Creation in Canine Vocal Folds
- PMID: 37096749
- PMCID: PMC10754041
- DOI: 10.1002/lary.30713
Ultrafast Laser Microlaryngeal Surgery for In Vivo Subepithelial Void Creation in Canine Vocal Folds
Abstract
Background/objectives: Tightly-focused ultrafast laser pulses (pulse widths of 100 fs-10 ps) provide high peak intensities to produce a spatially confined tissue ablation effect. The creation of sub-epithelial voids within scarred vocal folds (VFs) via ultrafast laser ablation may help to localize injectable biomaterials to treat VF scarring. Here, we demonstrate the feasibility of this technique in an animal model using a custom-designed endolaryngeal laser surgery probe.
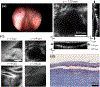
Methods: Unilateral VF mucosal injuries were created in two canines. Four months later, ultrashort laser pulses (5 ps pulses at 500 kHz) were delivered via the custom laser probe to create sub-epithelial voids of ~3 × 3-mm2 in both healthy and scarred VFs. PEG-rhodamine was injected into these voids. Ex vivo optical imaging and histology were used to assess void morphology and biomaterial localization.
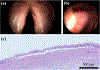
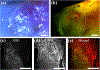
Results: Large sub-epithelial voids were observed in both healthy and scarred VFs immediately following in vivo laser treatment. Two-photon imaging and histology confirmed ~3-mm wide subsurface voids in healthy and scarred VFs of canine #2. Biomaterial localization within a void created in the scarred VF of canine #2 was confirmed with fluorescence imaging but was not visualized during follow-up two-photon imaging. As an alternative, the biomaterial was injected into the excised VF and could be observed to localize within the void.
Conclusions: We demonstrated sub-epithelial void formation and the ability to inject biomaterials into voids in a chronic VF scarring model. This proof-of-concept study provides preliminary evidence towards the clinical feasibility of such an approach to treating VF scarring using injectable biomaterials.
Level of evidences: N/A Laryngoscope, 133:3042-3048, 2023.
Keywords: biomaterials; endoscopy; microlaryngeal surgery; phonosurgery.
© 2023 The American Laryngological, Rhinological and Otological Society, Inc.
Conflict of interest statement
Figures

Similar articles
-
In vivo hamster cheek pouch subepithelial ablation, biomaterial injection, and localization: pilot study.J Biomed Opt. 2022 Aug;27(8):080501. doi: 10.1117/1.JBO.27.8.080501. J Biomed Opt. 2022. PMID: 36008882 Free PMC article.
-
Ultrafast laser surgery probe for sub-surface ablation to enable biomaterial injection in vocal folds.Sci Rep. 2022 Nov 29;12(1):20554. doi: 10.1038/s41598-022-24446-5. Sci Rep. 2022. PMID: 36446830 Free PMC article.
-
Parameters affecting ultrafast laser microsurgery of subepithelial voids for scar treatment in vocal folds.J Biomed Opt. 2013 Nov;18(11):118001. doi: 10.1117/1.JBO.18.11.118001. J Biomed Opt. 2013. PMID: 24193950
-
Vocal fold scars: current concepts and future directions. Consensus report of the Phonosurgery Committee of the European Laryngological Society.Eur Arch Otorhinolaryngol. 2013 Sep;270(9):2491-507. doi: 10.1007/s00405-013-2498-9. Epub 2013 Apr 21. Eur Arch Otorhinolaryngol. 2013. PMID: 23605306 Review.
-
Bioreactors for Vocal Fold Tissue Engineering.Tissue Eng Part B Rev. 2022 Feb;28(1):182-205. doi: 10.1089/ten.TEB.2020.0285. Epub 2021 Mar 17. Tissue Eng Part B Rev. 2022. PMID: 33446061 Free PMC article. Review.
Cited by
-
Preclinical Vocal Fold and Airway Injury Models: A Scoping Review.Laryngoscope Investig Otolaryngol. 2025 Jul 31;10(4):e70173. doi: 10.1002/lio2.70173. eCollection 2025 Aug. Laryngoscope Investig Otolaryngol. 2025. PMID: 40746632 Free PMC article. Review.
-
Emerging ultrafast technologies in biotechnology.3 Biotech. 2025 May;15(5):142. doi: 10.1007/s13205-025-04309-2. Epub 2025 Apr 24. 3 Biotech. 2025. PMID: 40292246 Review.
References
-
- Ramig LO, Verdolini K. Treatment efficacy: voice disorders. J Speech Lang Hear Res. 1998;41(1):S101–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

