Generation of Rh D-negative blood using CRISPR/Cas9
- PMID: 37096780
- PMCID: PMC10623963
- DOI: 10.1111/cpr.13486
Generation of Rh D-negative blood using CRISPR/Cas9
Abstract
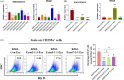
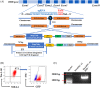
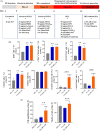
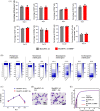
Blood supply shortages, especially the shortage of rare blood types, threaten the current medical system. Research on stem cells has shed light on in vitro blood cell manufacturing. The in vitro production of universal red blood cells (RBCs) from induced pluripotent stem cells (iPSCs) has become the focus of transfusion medicine. To obtain O-type Rh D-negative blood, we developed O-type Rh D-negative human (h)iPSCs using homology-directed repair (HDR)-based CRISPR/Cas9. HuAiPSCs derived from human umbilical arterial endothelial cells and showing haematopoietic differentiation preferences were selected for gene modification. Guide RNAs (gRNAs) were selected, and a donor template flanked by gRNA-directed homologous arms was set to introduce a premature stop code to RHD exon 2. CRISPR/Cas9 gene editing has resulted in the successful generation of an RHD knockout cell line. The HuAiPSC-A1-RHD-/- cell line was differentiated into haematopoietic stem/progenitor cells and subsequently into erythrocytes in the oxygen concentration-optimized differentiation scheme. HuAiPSC-A1-RHD-/- derived erythrocytes remained positive for the RBC markers CD71 and CD235a. These erythrocytes did not express D antigen and did not agglutinate in the presence of anti-Rh D reagents. In conclusion, taking the priority of haematopoietic preference hiPSCs, the HDR-based CRISPR/Cas9 system and optimizing the erythroid-lineage differentiation protocol, we first generated O-type Rh D-negative universal erythrocytes from RHD knockout HuAiPSCs. Its production is highly efficient and shows great potential for clinical applications.
© 2023 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Generation of 'designer erythroblasts' lacking one or more blood group systems from CRISPR/Cas9 gene-edited human-induced pluripotent stem cells.J Cell Mol Med. 2021 Oct;25(19):9340-9349. doi: 10.1111/jcmm.16872. Epub 2021 Sep 21. J Cell Mol Med. 2021. PMID: 34547166 Free PMC article.
-
Footprint-free gene mutation correction in induced pluripotent stem cell (iPSC) derived from recessive dystrophic epidermolysis bullosa (RDEB) using the CRISPR/Cas9 and piggyBac transposon system.J Dermatol Sci. 2020 Jun;98(3):163-172. doi: 10.1016/j.jdermsci.2020.04.004. Epub 2020 Apr 24. J Dermatol Sci. 2020. PMID: 32376152
-
Efficient, footprint-free human iPSC genome editing by consolidation of Cas9/CRISPR and piggyBac technologies.Nat Protoc. 2017 Jan;12(1):88-103. doi: 10.1038/nprot.2016.152. Epub 2016 Dec 8. Nat Protoc. 2017. PMID: 27929521 Free PMC article.
-
Genome Editing in Induced Pluripotent Stem Cells using CRISPR/Cas9.Stem Cell Rev Rep. 2018 Jun;14(3):323-336. doi: 10.1007/s12015-018-9811-3. Stem Cell Rev Rep. 2018. PMID: 29623532 Review.
-
Genome Engineering for Stem Cell Transplantation.Exp Clin Transplant. 2019 Jan;17(Suppl 1):31-37. doi: 10.6002/ect.MESOT2018.L34. Exp Clin Transplant. 2019. PMID: 30777520 Review.
Cited by
-
KDM4B modulates autocrine IL6 in erythroblasts to prevent ineffective erythropoiesis.Leukemia. 2025 May;39(5):1228-1242. doi: 10.1038/s41375-025-02559-w. Epub 2025 Mar 12. Leukemia. 2025. PMID: 40074853
-
PROTAC-mediated vimentin degradation promotes terminal erythroid differentiation of pluripotent stem cells.Stem Cell Res Ther. 2024 Sep 18;15(1):310. doi: 10.1186/s13287-024-03910-1. Stem Cell Res Ther. 2024. PMID: 39294765 Free PMC article.
-
3' UTR-truncated HMGA2 promotes erythroblasts production from human embryonic stem cells.Stem Cells Transl Med. 2025 Jan 17;14(1):szaf001. doi: 10.1093/stcltm/szaf001. Stem Cells Transl Med. 2025. PMID: 39912395 Free PMC article.
-
Enhancing terminal erythroid differentiation in human embryonic stem cells through TRIB3 overexpression.Heliyon. 2024 Sep 5;10(18):e37463. doi: 10.1016/j.heliyon.2024.e37463. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309892 Free PMC article.
References
MeSH terms
Grants and funding
- 202002030025/Science and Technology Program of Guangzhou, China
- 32200589/National Nature Science Foundation of China
- 201904010378/The Guangzhou Scientific Research Program
- 2017YFA0103100/The National Key Research and Development Program of China
- 2017YFA0103103/The National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources

