Interventions for dialysis patients with hepatitis C virus (HCV) infection
- PMID: 37096802
- PMCID: PMC10130818
- DOI: 10.1002/14651858.CD007003.pub3
Interventions for dialysis patients with hepatitis C virus (HCV) infection
Abstract
Background: Hepatitis C virus (HCV) infection is common in chronic kidney disease (CKD) patients on dialysis, causes chronic liver disease, may increase the risk of death, and impacts kidney transplant outcomes. Direct-acting antivirals have replaced interferons because of better efficacy and tolerability. This is an update of a review first published in 2015.
Objectives: We aimed to look at the benefits and harms of interventions for HCV in CKD patients on dialysis: death, disease relapse, treatment response/discontinuation, time to recovery, quality of life (QoL), cost-effectiveness, and adverse events. We aimed to study comparisons of available interventions, compared with placebo, control, with each other and with newer treatments.
Search methods: We searched the Cochrane Kidney and Transplant's Specialised Register to 23 February 2023 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE and EMBASE, handsearching conference proceedings, and searching the International Clinical Trials Register Portal (ICTRP) and ClinicalTrials.gov.
Selection criteria: Randomised controlled trials (RCTs), quasi-RCTs, first period of randomised cross-over studies on interventions for HCV in CKD on dialysis were considered.
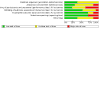
Data collection and analysis: Summary estimates of effect were obtained using a random-effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI). Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
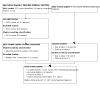
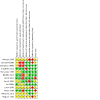
Main results: Three studies were included in this update, therefore 13 studies (997 randomised participants) met our inclusion criteria. Overall, the risk of bias was judged low in seven studies, unclear in four, low to unclear in one, and high in one study. Interventions included standard interferon, pegylated (PEG) interferon, standard or PEG interferon plus ribavirin; direct-acting antivirals, and direct-acting antivirals plus PEG interferon plus ribavirin. Compared to placebo or control, standard interferon may make little or no difference to death (5 studies, 134 participants: RR 0.89, 95% CI 0.06 to 13.23) or relapse (low certainty evidence), probably improves end-of-treatment response (ETR) (5 studies, 132 participants: RR 8.62, 95% CI 3.03 to 24.55; I² = 0%) (moderate certainty evidence), and probably makes little or no difference to sustained virological response (SVR) (4 studies, 98 participants: RR 3.25, 95% CI 0.81 to 13.07; I² = 53%), treatment discontinuation (4 studies, 116 participants: RR 4.59, 95% CI 0.49 to 42.69; I² = 63%), and adverse events (5 studies, 143 participants: RR 3.56, 95% CI 0.98 to 13.01; I² = 25%) (moderate certainty evidence). In low certainty evidence, PEG interferon (1 study, 50 participants) may improve ETR (RR 1.53, 95% CI 1.09 to 2.15) but may make little or no difference to death (RR 0.33, 95% CI 0.01 to 7.81), SVR (RR 2.40, 95% CI 0.99 to 5.81), treatment discontinuation (RR 0.11, 95% CI 0.01 to 1.96), adverse events (RR 0.11, 95% CI 0.01 to 1.96) and relapses (21/38 relapsed) (RR 0.72, 95% CI 0.41 to 1.25) compared to standard interferon. In moderate certainty evidence, high-dose PEG interferon (alpha-2a and alpha-2b) may make little or no difference to death (2 studies, 97 participants: RR 4.30, 95% CI 0.76 to 24.33; I² = 0%), ETR (RR 1.42, 95% CI 0.51 to 3.90; I² = 20%), SVR (RR 1.19, 95% CI 0.68 to 2.07; I² = 0%), treatment discontinuation (RR 1.20, 95% CI 0.63 to 2.28; I² = 0%) or adverse events (RR 1.05, 95% CI 0.61 to 1.83; I² = 27%) compared to low-dose PEG interferon. High-dose PEG interferon may make little or no difference to relapses (1 study, 43 participants: RR 1.11, 95% CI 0.45 to 2.77; low certainty evidence). There were no significant subgroup differences. Standard interferon plus ribavirin may lead to higher treatment discontinuation (1 study, 52 participants: RR 2.97, 95% CI 1.19 to 7.36; low certainty evidence) compared to standard interferon alone. In low certainty evidence, PEG interferon plus ribavirin (1 study, 377 participants) may improve SVR (RR 1.80, 95% CI 1.46 to 2.21), reduce relapses (RR 0.33, 95% CI 0.23 to 0.48), slightly increase the number with adverse events (RR 1.10, 95% CI 1.01 to 1.19), and may make little or no difference to ETR (RR 1.01, 95% CI 0.94 to 1.09) compared to PEG interferon alone. The evidence is very uncertain about the effect of PEG interferon plus ribavirin on treatment discontinuation (RR 1.71, 95% CI 0.69 to 4.24) compared to PEG interferon alone. One study reported grazoprevir plus elbasvir improved ETR (173 participants: RR 174.99, 95% CI 11.03 to 2775.78; low certainty evidence) compared to placebo. It is uncertain whether telaprevir plus ribavirin (high versus low initial dose) plus PEG interferon for 24 versus 48 weeks (1 study, 35 participants) improves ETR (RR 1.02, 95% CI 0.67 to 1.56) or SVR (RR 1.02, 95% CI 0.67 to 1.56) because the certainty of the evidence is very low. Data on QoL, cost-effectiveness, cardiovascular outcomes and peritoneal dialysis were not available.
Authors' conclusions: In dialysis patients with HCV infection grazoprevir plus elbasvir probably improves ETR. There is no difference in ETR or SVR for combinations of telaprevir, ribavirin and PEG interferon given for different durations and doses. Though no longer in use, PEG interferon was more effective than standard interferon for ETR but not SVR. Increasing doses of PEG interferon did not improve responses. The addition of ribavirin to PEG interferon may result in fewer relapses, higher SVR, and higher numbers with adverse events.
Trial registration: ClinicalTrials.gov NCT02092350 NCT00491244 NCT02806505 NCT00172809.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Attur Ravindra Prabhu: no relevant interests were disclosed
Indu Ramachandra Rao: no relevant interests were disclosed
Shankar Prasad Nagaraju: no relevant interests were disclosed
Eti Rajwar: no relevant interests were disclosed
Bhumika T Venkatesh: no relevant interests were disclosed
Sreekumaran Nair N: no relevant interests were disclosed
Ganesh Pai: no relevant interests were disclosed
Nageswara P Reddy: no relevant interests were disclosed
Deepak Suvarna: no relevant interests were disclosed
Figures


























Update of
-
Interventions for dialysis patients with hepatitis C virus (HCV) infection.Cochrane Database Syst Rev. 2015 Aug 19;2015(8):CD007003. doi: 10.1002/14651858.CD007003.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2023 Apr 25;4:CD007003. doi: 10.1002/14651858.CD007003.pub3. PMID: 26287983 Free PMC article. Updated.
Similar articles
-
Interventions for dialysis patients with hepatitis C virus (HCV) infection.Cochrane Database Syst Rev. 2015 Aug 19;2015(8):CD007003. doi: 10.1002/14651858.CD007003.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2023 Apr 25;4:CD007003. doi: 10.1002/14651858.CD007003.pub3. PMID: 26287983 Free PMC article. Updated.
-
Pharmacological interventions for acute hepatitis C infection.Cochrane Database Syst Rev. 2018 Dec 3;12(12):CD011644. doi: 10.1002/14651858.CD011644.pub3. Cochrane Database Syst Rev. 2018. PMID: 30521693 Free PMC article.
-
Potassium binders for chronic hyperkalaemia in people with chronic kidney disease.Cochrane Database Syst Rev. 2020 Jun 26;6(6):CD013165. doi: 10.1002/14651858.CD013165.pub2. Cochrane Database Syst Rev. 2020. PMID: 32588430 Free PMC article.
-
Psychosocial interventions for preventing and treating depression in dialysis patients.Cochrane Database Syst Rev. 2019 Dec 2;12(12):CD004542. doi: 10.1002/14651858.CD004542.pub3. Cochrane Database Syst Rev. 2019. PMID: 31789430 Free PMC article.
-
Pharmacological interventions versus placebo, no treatment or usual care for osteoporosis in people with chronic kidney disease stages 3-5D.Cochrane Database Syst Rev. 2021 Jul 7;7(7):CD013424. doi: 10.1002/14651858.CD013424.pub2. Cochrane Database Syst Rev. 2021. PMID: 34231877 Free PMC article.
Cited by
-
Virtual high-throughput screening: Potential inhibitors targeting aminopeptidase N (CD13) and PIKfyve for SARS-CoV-2.Open Life Sci. 2023 Jul 7;18(1):20220637. doi: 10.1515/biol-2022-0637. eCollection 2023. Open Life Sci. 2023. PMID: 37426619 Free PMC article.
References
References to studies included in this review
Alfurayh 2000 {published data only}
-
- Alfurayh O, Chaaban A, Pall A, Al Mutawa N, Ellis M, Al Meshari K, et al. Long-term follow up of haemodialysis (HD) patients with chronic hepatitis C (HCV) infection - favorable outcome with a-interferon (IFN) followed by kidney transplantation [abstract no: M221]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):110. [CENTRAL: CN-00509051]
-
- Alfurayh O, Chaaban A, Pall A, Ellis M, Chaudry T, Almeshari K. Randomised controlled trial of interferon-alpha (IFNalpha) in patients with chronic hepatitis C (HCV) on haemodialysis (HD) [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A106. [CENTRAL: CN-00497910]
-
- Alfurayh OI, Chaaban AM, Pall AA, Ellis ME, Almeshari K, Al Quaiz MN, et al. IFN-alpha for chronic hepatitis C infection (HCV) in haemodialysis (HD) patients - a prospective, randomised, controlled study [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):255A. [CENTRAL: CN-00517117]
Campistol 1996 {published data only}
-
- Campistol JM, Casellas J, Cuevas X, Latorre B, Martínez J, Modol J, et al. Efficacy and tolerability of a-2b interferon in the treatment of virus C chronic hepatitis in an hemodialysis population [abstract no: 309] [Eficacia y tolerancia del interferon a-2b en el tratamiento de la hepatitis cronica por virus C (VHC) en la población en hemodialisis (HD)]. Nefrología 1996;16(Suppl 1):80. [CENTRAL: CN-00400461]
Campistol 1999a {published data only}
-
- Campistol JM, Esforzado N, Martinez J, Rosello L, Veciana L, Modol J, et al. Efficacy and tolerance of interferon-alpha(2b) in the treatment of chronic hepatitis C virus infection in haemodialysis patients. Pre- and post-renal transplantation assessment. Nephrology Dialysis Transplantation 1999;14(11):2704-9. [MEDLINE: ] - PubMed
C‐SURFER 2015 {published data only}
-
- Arduino JM, Zhang B, Jackson B, Roth D, Bruchfeld A, khawaja S, et al. Impact of grazoprevir plus elbasvir on health-related quality of life in patients with hepatitis C virus genotype 1 infection and chronic kidney disease stages 4 and 5 [abstract no: TH-PO667]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):242A.
-
- Barr E, Roth D, Bruchfeld A, Martin P, Nelson DR, Silva M, et al. Elbasvir (EBR)/grazoprevir (GZR) treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease stage 4/5: final results of the C-SURFER phase 3 study [abstract]. Journal of Gastroenterology & Hepatology 2016;31(Suppl 2):65-6. [EMBASE: 612983742]
-
- Bruchfeld A, Roth D, Martin P, Nelson D, Stanislas P, Londono MC, et al. Elbasvir/Grazoprevir (EBR/GZR) treatment of hepatitis C Virus (HCV) infection in patients with chronic kidney disease (CKD) stage 4/5: clinical, virological, and health-related quality of life outcomes in the C-SURFER study [abstract]. Swiss Medical Weekly 2016;146(Suppl 221):23S. [EMBASE: 626845823]
-
- Bruchfeld A, Roth D, Martin P, Nelson DR, Pol S, Londono MC, et al. Elbasvir plus grazoprevir in patients with hepatitis C virus infection and stage 4-5 chronic kidney disease: clinical, virological, and health-related quality-of-life outcomes from a phase 3, multicentre, randomised, double-blind, placebo-controlled trial. The Lancet. Gastroenterology & Hepatology 2017;2(8):585-94. [MEDLINE: ] - PubMed
-
- Bruchfeld A, Roth D, Nelson D, Liapakis AM, Silva M, Monsour H, et al. C-surfer: Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and chronic kidney disease [abstract no: FP267]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii156. [EMBASE: 72206687] - PubMed
Fernandez 1997 {published data only}
-
- Fernandez JL, Rendo P, Pino N, Nephrologists' Group for the Study of HCV Infection, Viola L. A double-blind controlled trial of recombinant interferon alfa in hemodialysis patients with chronic hepatitis C [abstract]. Hepatology 1995;22(4 (Pt 2)):116A. [CENTRAL: CN-00220972] - PubMed
-
- Fernandez JL, Rendo P, Pino N, Nephrologists' Group for the Study of HCV, Viola L, Cusumano A. A controlled trial of interferon alfa 2b in hemodialysis patients with chronic hepatitis C [abstract no: A0989]. Journal of the American Society of Nephrology 1996;7(9):1446. [CENTRAL: CN-00445323]
-
- Fernandez JL, Rendo P, Pino N, Viola L. A double-blind controlled trial of recombinant interferon-alpha 2b in haemodialysis patients with chronic hepatitis C virus infection and abnormal aminotransferase levels. Nephrologists' Group for the Study of HCV infection. Journal of Viral Hepatitis 1997;4(2):113-9. [MEDLINE: ] - PubMed
HELPER 2013 {published data only}
-
- Liu CH, Huang CF, Liu CJ, Dai CY, Liang CC, Huang JF, et al. Peginterferon alfa-2a with or without low dose ribavirin for hemodialysis patients with hepatitis C virus genotype 2 infection: a randomized trial [abstract no: 1851]. Hepatology 2013;58(4 Suppl 1):1096A. [EMBASE: 71237851]
-
- Liu CH, Huang CF, Liu CJ, Dai CY, Liang CC, Huang JF, et al. Pegylated interferon-alpha2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: a randomized trial. Annals of Internal Medicine 2013;159(11):729-38. [MEDLINE: ] - PubMed
-
- Liu CH, Liang CC, Su TH, Hung PH, Tsai HB, Liu CJ, et al. Peginterferon alfa-2a plus low dose ribavirin versus peginterferon alfa-2a monotherapy for dialysis patients with hepatitis C virus genotype 1 infection: a randomized trial [abstract no: 1722]. Hepatology 2012;56(4 Suppl 1):993A. [EMBASE: 70943304]
-
- Liu CH, Liu CJ, Huang CF, Lin JW, Dai CY, Liang CC, et al. Peginterferon alfa-2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 2 receiving haemodialysis: a randomised trial. Gut 2015;64(2):303-11. [MEDLINE: ] - PubMed
HELPS 2011 {published data only}
-
- Peck-Radosavljevic M, Boletis J, Besisik F, Lucia Ferraz M, Alric L, Samuel D, et al. Low-dose peginterferon alfa-2A (40KD) (PEGASYS®) to treat hepatitis C infected end-stage renal disease patients undergoing haemodialysis: final study results [abstract no: 999]. Journal of Hepatology 2008;48(Suppl 2):S374. [CENTRAL: CN-00653069]
-
- Peck-Radosavljevic M, Boletis J, Besisik F, Lucia Ferraz M, Alric L, Samuel D, et al. Low-dose peginterferon alfa-2a is safe and produces a sustained virologic response in patients with chronic hepatitis C and end-stage renal disease. Clinical Gastroenterology & Hepatology 2011;9(3):242-8. [MEDLINE: ] - PubMed
-
- Peck-Radosavljevic M, Boletis J, Besisik F, Lucia Ferraz M, Alric L, Samuel D, et al. Use of low-dose peginterferon alfa-2a (40 kD) (Pegasys) to treat hepatitis C infected end-stage renal disease patients undergoing haemodialysis: interim results from a randomised study [abstract no: 628]. Journal of Hepatology 2007;46(Suppl 1):S237-8. [CENTRAL: CN-00716095]
Huraib 2001 {published data only}
-
- Huraib S, Iqbal A, Tanimu D, Abdullah A. Sustained virological and histological response with pretransplant interferon therapy in renal transplant patients with chronic viral hepatitis C. American Journal of Nephrology 2001;21(6):435-40. [MEDLINE: ] - PubMed
Liu 2008a {published data only}
-
- Liu CH, Liang CC, Lin JW, Chen SI, Tsai HB, Chang CS, et al. Pegylated interferon alpha-2a versus standard interferon alpha-2a for treatment-naive dialysis patients with chronic hepatitis C: a randomised study. Gut 2008;57(4):525-30. [MEDLINE: ] - PubMed
Luxon 2005 {published data only}
-
- Luxon BA, Muir AJ, Heneghan MA. Safety and tolerability of pegylated interferon with or without low dose ribavirin for treatment of hepatitis C in hemodialysis patients [abstract no: 1281]. Hepatology 2005;42(4 Suppl 1):703-4A. [CENTRAL: CN-00581669]
Russo 2006 {published data only}
-
- Russo MW, Ghalib R, Sigal S, Joshi V. Randomized trial of pegylated interferon alpha-2b monotherapy in haemodialysis patients with chronic hepatitis C. Nephrology Dialysis Transplantation 2006;21(2):437-43. [MEDLINE: ] - PubMed
-
- Russo MW, Ghalib R, Sigal SH, Joshi V, Detwiler R, Andreoni K, et al. A multi-center randomized trial of pegylated interferon alfa-2B monotherapy (PEG-INTRON) in patients with chronic hepatitis C and end stage kidney disease on dialysis [abstract no: 540]. Hepatology 2004;40(4 Suppl 1):399A. [CENTRAL: CN-00507218]
TARGET C 2013 {published data only}
-
- Basu P, Shah N, Siriki R, Rahaman M, Farhat S. Telaprevir with adjusted dose of ribavirin in naive CHC-G1: efficacy and treatment in CHC in hemodialysis population target C trial: a placebo randomized control clinical trial [abstract no: 513]. American Journal of Gastroenterology 2013;108(Suppl 1):S152. [EMBASE: 71220810]
-
- Basu P, Siriki R, Shah NJ, Farhat S, Mittimani K, Atluri S, et al. Telaprevir with adjusted dose of ribavirin in naive CHC-G1: Efficacy and treatment in CHC in hemodialysis population. Target C trial-A placebo randomized control clinical trial [abstract no: 517]. Gastroenterology 2013;144(5 Suppl 1):S950. [EMBASE: 71119328]
-
- Basu PP, Shah NJ, Ashfique, Farhat S, Siriki R. Telaprevir with adjusted dose of ribavirin in naive CHC-G1: efficacy and treatment in CHC in hemodialysis population. Target C trial-a placebo randomized control clinical trial [abstract no: P196]. Surgical Endoscopy 2014;28(1 Suppl):S362. [EMBASE: 71480241]
-
- Basu PP, Shah NJ, Siriki R, Rahaman M. Telaprevir with adjusted dose of ribavirin in Naive CHC-G1: Efficacy and treatment in CHC in hemodialysis population. TARGET C Trial-a placebo randomized control clinical trial [abstract no: P-298]. Liver Transplantation 2014;20(Suppl 1):S244. [EMBASE: 71562627]
-
- Basu PP, Sinki R, Shah NJ, Farhat S, Mittimani K, Atluri S, et al. Telaprevir with adjusted dose of ribavirin in naive CHC-G1: efficacy and treatment in CHC in hemodialysis population. TARGET C (RCT) [abstract no: 67]. Journal of Hepatology 2013;58(Suppl 1):S30-1. [EMBASE: 71054340]
Tuglular 2001 {published data only}
-
- Tuglular S, Karadayi h, Karakullukcu F, Erman M, Yuksel S, Celik M, et al. Preliminary results of interferon compared to interferon combined with ribavirin in the treatment of chronic HCV in patients on chronic hemodialysis [abstract no: A2147]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):416-7A. [CENTRAL: CN-00543899]
References to studies excluded from this review
Ellis 1993 {published data only}
-
- Ellis ME, Alfurayh O, Halim MA, Sieck JO, Ali MA, Bernvil SS, et al. Chronic non-A, non-B hepatitis complicated by end-stage renal failure treated with recombinant interferon alpha. Journal of Hepatology 1993;18(2):210-6. [MEDLINE: ] - PubMed
Simon 1984 {published data only}
-
- Simon N. Prevention of non-A, non-B hepatitis in haemodialysis patients by hepatitis B immunoglobulin. Lancet 1984;2(8410):1047. [MEDLINE: ] - PubMed
References to studies awaiting assessment
Muzammil 2022 {published data only}
-
- Muzammil M, Tahir M, Ullah N, Arif MM, Rasheeq T, Ather MM. Comparison of efficacy of daily sofosbuvir and declatasvir with alternate day sofosbuvir and declatasvir in hepatitis C patients on hemodialysis in Pakistani population. Pakistan Journal of Medical & Health Sciences 2022;16(8):264-6. [CENTRAL: CN-02468336] [DOI: 10.53350/pjmhs22168264] - DOI
Additional references
AASLD/IDSA HCV Guidance Panel 2020
-
- Ghany MG, Morgan TR, AASLD/IDSA HCV Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020;71(2):686-721. [DOI: ] - PMC - PubMed
Adinolfi 2018
-
- Adinolfi LE, Nevola R, Guerrera B, D'Alterio G, Marrone A, Giordano M, et al. Hepatitis C virus clearance by direct-acting antiviral treatments and impact on insulin resistance in chronic hepatitis C patients. Journal of Gastroenterology & Hepatology 2018;33(7):1379-82. [MEDLINE: ] - PubMed
Alavian 2010
-
- Alavian SM, Tabatabaei SV. Meta-analysis of factors associated with sustained viral response in patients on hemodialysis treated with standard or pegylated interferon for hepatitis C infection. Iranian Journal of Kidney Diseases 2010;4(3):181-94. [MEDLINE: ] - PubMed
Arase 2009
-
- Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology 2009;49(3):739-44. [MEDLINE: ] - PubMed
Ayaz 2008
Baden 2012
-
- Baden LR, Dolin R. Antiviral chemotherapy excluding antiretroviral drugs. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors(s). Harrison's Principles of Internal Medicine. 18th edition. Vol. 1. New York: McGraw-Hill, 2012:1447. [ISBN: 978-0-07-163244-7]
Bergman 2005
-
- Bergman S, Accortt N, Turner A, Glaze J. Hepatitis C infection is acquired pre-ESRD. American Journal of Kidney Diseases 2005;45(4):684-9. [MEDLINE: ] - PubMed
Bravo 2016
-
- Bravo Zuñiga JI, Loza Munárriz C, López‐Alcalde J. Isolation as a strategy for controlling the transmission of hepatitis C virus (HCV) infection in haemodialysis units. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD006420. [DOI: 10.1002/14651858.CD006420.pub2] - DOI - PMC - PubMed
Bruchfeld 2001
-
- Bruchfeld A, Stahle L, Andersson J, Schvarcz R. Ribavirin treatment in dialysis patients with chronic hepatitis C virus infection--a pilot study. Journal of Viral Hepatitis 2001;8(4):287–92. [MEDLINE: ] - PubMed
Bruchfeld 2017
-
- Bruchfeld A, Roth D, Martin P, Nelson DR, Pol S, Londono MC, et al. Elbasvir plus grazoprevir in patients with hepatitis C virus infection and stage 4-5 chronic kidney disease: clinical, virological, and health-related quality-of-life outcomes from a phase 3, multicentre, randomised, double-blind, placebo-controlled trial. The Lancet. Gastroenterology & Hepatology 2017;2(8):585-94. [MEDLINE: ] - PubMed
Burra 2006
-
- Burra P, Buda A, Livi U, Rigotti P, Zanus G, Calabrese F, et al. Occurrence of post-transplant lymphoproliferative disorders among over thousand adult recipients: any role for hepatitis C infection? European Journal of Gastroenterology & Hepatology 2006;18(10):1065-70. [MEDLINE: ] - PubMed
Carrat 2019
-
- Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 2019;393(10179):1453-64. [MEDLINE: ] - PubMed
Chandra 2004
-
- Chandra M, Khaja MN, Hussain MM, Poduri CD, Farees N, Habeeb MA, et al. Prevalence of hepatitis B and hepatitis C viral infections in Indian patients with chronic renal failure. Intervirology 2004;47(6):374-6. [MEDLINE: ] - PubMed
Chaparro 2009
-
- Chaparro M, Trapero-Marugan M, Moreno-Otero R, Gisbert JP. Azathioprine plus ribavirin treatment and pancytopenia. Alimentary Pharmacology & Therapeutics 2009;30(9):962-3. [MEDLINE: ] - PubMed
Choo 1989
-
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989;244(4902):359-62. [MEDLINE: ] - PubMed
Cruzado 2001
-
- Cruzado JM, Carrera M, Torras J, Grinyó JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. American Journal of Transplantation 2001;1(2):171-8. [MEDLINE: ] - PubMed
Dienstag 2012a
-
- Dienstag JL. Chapter 203: Acute viral hepatitis. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors(s). Harrison's Principles of Internal Medicine. 18th edition. Vol. 2. New York: McGraw-Hill, 2012:2542-8. [ISBN: 978-0-07-174887-2]
Dienstag 2012b
-
- Dienstag JL. Chapter 306: Chronic hepatitis. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors(s). Harrison's Principles of Internal Medicine. 18th edition. Vol. 2. New York: McGraw-Hill, 2012:2578-84. [ISBN: 978-0-07-174887-2]
Dienstag 2022
-
- Dienstag JL. Chapter 341: Chronic hepatitis. In: Loscalzo J, Fauci KD, Hauser S, Longo D, Jameson JL, editors(s). Harrison's Principles of Internal Medicine. 21st edition. New York, NY: McGraw-Hill Education, 2022:2591-616. [ISBN: 9781264268504]
Fabrizi 2003
-
- Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P. Meta-analysis: interferon for the treatment of chronic hepatitis C in dialysis patients. Alimentary Pharmacology & Therapeutics 2003;18(11-12):1071-81. [MEDLINE: ] - PubMed
Fabrizi 2004
-
- Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: effect of hepatitis C virus infection on mortality in dialysis. Alimentary Pharmacology & Therapeutics 2004;20(11-12):1271-7. [MEDLINE: ] - PubMed
Fabrizi 2005
-
- Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. American Journal of Transplantation 2005;5(10):2433-40. [MEDLINE: ] - PubMed
Fabrizi 2008a
-
- Fabrizi F, Ganeshan SV, Lunghi G, Messa P, Martin P. Antiviral therapy of hepatitis C in chronic kidney diseases: meta-analysis of controlled clinical trials. Journal of Viral Hepatitis 2008;15(8):600-6. [MEDLINE: ] - PubMed
Fabrizi 2008b
-
- Fabrizi F, Dixit V, Messa P, Martin P. Interferon monotherapy of chronic hepatitis C in dialysis patients: meta-analysis of clinical trials. Journal of Viral Hepatitis 2008;15(2):79-88. [MEDLINE: ] - PubMed
Fabrizi 2014
-
- Fabrizi F, Martin P, Dixit V, Messa P. Meta-analysis of observational studies: hepatitis C and survival after renal transplant. Journal of Viral Hepatitis 2014;21(5):314-24. [MEDLINE: ] - PubMed
Fabrizi 2019
-
- Fabrizi F, Dixit V, Messa P. Hepatitis C virus and mortality among patients on dialysis: A systematic review and meta-analysis. Clinics & Research in Hepatology & Gastroenterology 2019;43(3):244-54. [MEDLINE: ] - PubMed
Gane 2017
-
- Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. New England Journal of Medicine 2017;377(15):1448-55. [MEDLINE: ] - PubMed
Gomez 1999
-
- Gomez J, Martell M, Quer J, Cabot B, Esteban JI. Hepatitis C viral quasispecies. Journal of Viral Hepatitis 1999;6(1):3-16. [MEDLINE: ] - PubMed
Goodkin 2017
GRADE 2008
GRADE 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] - PubMed
Hammoud 1996
-
- Hammoud H, Haem J, Laurent B, Alamartine E, Diab N, Defilippis JP, et al. Glomerular disease during HCV infection in renal transplantation. Nephrology Dialysis Transplantation 1996;11 Suppl 4:54-5. [MEDLINE: ] - PubMed
Hassan 2000
-
- Hassan AA, Khalil R. Hepatitis C in dialysis patients in Egypt: relationship to dialysis duration, blood transfusion, and liver disease. Saudi Journal of Kidney Diseases & Transplantation 2000;11(1):72-3. [MEDLINE: ] - PubMed
Hayes 2021
-
- Hayes KN, Burkard T, Weiler S, Tadrous M, Burden AM. Global adverse events reported for direct-acting antiviral therapies for the treatment of hepatitis C: an analysis of the World Health Organization VigiBase. European Journal of Gastroenterology & Hepatology 2021;33(1S Suppl 1):e1017-21. [MEDLINE: ] - PMC - PubMed
Higgins 2003
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Houglum 1983
-
- Houglum JE. Interferon: mechanisms of action and clinical value. Clinical Pharmacy 1983;2(1):20-8. [MEDLINE: ] - PubMed
Iliescu 2020
Jadoul 2019
-
- Jadoul M, Bieber BA, Martin P, Akiba T, Nwankwo C, Arduino JM, et al. Prevalence, incidence, and risk factors for hepatitis C virus infection in hemodialysis patients. Kidney International 2019;95(4):939-47. [MEDLINE: ] - PubMed
Jakobsen 2017
Kalidindi 2020
KDIGO 2018
Lawitz 2019
Li 2017
-
- Li T, Qu Y, Guo Y, Wang Y, WL. Efficacy and safety of direct-acting antivirals-based antiviral therapies for hepatitis C virus patients with stage 4-5 chronic kidney disease: a meta-analysis. Liver International 2017;37(7):974-81. [MEDLINE: ] - PubMed
Magone 1995
Majd Jabbari 2021
Moreno 2004
-
- Moreno A, Quereda C, Moreno L, Perez-Elias MJ, Muriel A, Casado JL, et al. High rate of didanosine-related mitochondrial toxicity in HIV/HCV-coinfected patients receiving ribavirin. Antiviral Therapy 2004;9(1):133-8. [MEDLINE: ] - PubMed
Nahon 2017
-
- Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications [Erratum in: Gastroenterology. 2021 Jul;161(1):377; PMID: 30817897]. Gastroenterology 2017;152(1):142-56. [MEDLINE: ] - PubMed
Nakayama 2000
-
- Nakayama E, Akiba T, Marumo F, Sato C. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. Journal of the American Society of Nephrology 2000;11(10):1896-902. [MEDLINE: ] - PubMed
Neumann 1998
-
- Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 1998;282(5386):103-7. [MEDLINE: ] - PubMed
NKF‐K/DOQI 2002
-
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases 2002;39(2 Suppl 1):S1-266. [MEDLINE: ] - PubMed
Polaris Observatory HCV Collaborators 2017
-
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. The Lancet. Gastroenterology & Hepatology 2017;2(3):161-76. [MEDLINE: ] - PubMed
Poordad 2012
-
- Poordad F, Dieterich D. Treating hepatitis C: current standard of care and emerging direct-acting antiviral agents. Journal of Viral Hepatitis 2012;19(7):449-64. [MEDLINE: ] - PubMed
Rahnavardi 2008
-
- Rahnavardi M, Hosseini Moghaddam SM, Alavian SM. Hepatitis C in hemodialysis patients: current global magnitude, natural history, diagnostic difficulties, and preventive measures. American Journal of Nephrology 2008;28(4):628-40. [MEDLINE: ] - PubMed
Rostami 2011
-
- Rostami Z, Nourbala MH, Alavian SM, Bieraghdar F, Jahani Y, Einollahi B. The impact of Hepatitis C virus infection on kidney transplantation outcomes: A systematic review of 18 observational studies: The impact of HCV on renal transplantation. Hepatitis Monthly 2011;11(4):247-54. [MEDLINE: ] - PMC - PubMed
Russo 2003
-
- Russo MW, Goldsweig CD, Jacobson IM, Brown RS Jr. Interferon monotherapy for dialysis patients with chronic hepatitis C: an analysis of the literature on efficacy and safety. American Journal of Gastroenterology 2003;98(7):1610-5. [MEDLINE: ] - PubMed
Schunemann 2022a
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook.
Schunemann 2022b
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Shehadeh 2020
Simmons 2015
-
- Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clinical Infectious Diseases 2015;61(5):730-40. [MEDLINE: ] - PMC - PubMed
Thomas 2011
USP 2006
-
- United States Pharmacopeial Convention. USP D1. Volume 1, Drug Information for the Health Care Professional. Greenwood Village, CO: Thomson/MICROMEDEX, 2006.
Vial 1994
-
- Vial T, Descotes J. Clinical toxicity of the interferons. Drug Safety 1994;10(2):115-50. [MEDLINE: ] - PubMed
WHO 2016
-
- World Health Organization (2016). Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. https://apps.who.int/iris/handle/10665/246177 (accessed 18 February 2023).
Williams 1987
-
- Williams SJ, Baird-Lambert JA, Farrell GC. Inhibition of theophylline metabolism by interferon. Lancet 1987;2(8565):939-41. [MEDLINE: ] - PubMed
Wills 1990
-
- Wills RJ. Clinical pharmacokinetics of interferons. Clinical Pharmacokinetics 1990;19(5):390-9. [MEDLINE: ] - PubMed
Zhang 2008
References to other published versions of this review
Prabhu 2008
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

