Regulation of CD8 T-cell signaling, metabolism, and cytotoxic activity by extracellular lysophosphatidic acid
- PMID: 37096808
- PMCID: PMC10523933
- DOI: 10.1111/imr.13208
Regulation of CD8 T-cell signaling, metabolism, and cytotoxic activity by extracellular lysophosphatidic acid
Abstract
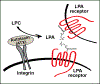
Lysophosphatidic acid (LPA) is an endogenous bioactive lipid that is produced extracellularly and signals to cells via cognate LPA receptors, which are G-protein coupled receptors (GPCRs). Mature lymphocytes in mice and humans express three LPA receptors, LPA2 , LPA5, and LPA6 , and work from our group has determined that LPA5 signaling by T lymphocytes inhibits specific antigen-receptor signaling pathways that ultimately impair lymphocyte activation, proliferation, and function. In this review, we discuss previous and ongoing work characterizing the ability of an LPA-LPA5 axis to serve as a peripheral immunological tolerance mechanism that restrains adaptive immunity but is subverted during settings of chronic inflammation. Specifically, LPA-LPA5 signaling is found to regulate effector cytotoxic CD8 T cells by (at least) two mechanisms: (i) regulating the actin-microtubule cytoskeleton in a manner that impairs immunological synapse formation between an effector CD8 T cell and antigen-specific target cell, thus directly impairing cytotoxic activity, and (ii) shifting T-cell metabolism to depend on fatty-acid oxidation for mitochondrial respiration and reducing metabolic efficiency. The in vivo outcome of LPA5 inhibitory activity impairs CD8 T-cell killing and tumor immunity in mouse models providing impetus to consider LPA5 antagonism for the treatment of malignancies and chronic infections.
Keywords: T cells; cytotoxic; cytotoxicity; lipid mediators; signal transduction cancer.
© 2023 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no competing interests.
Figures


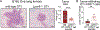
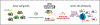

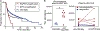
References
-
- Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta. 2008;1781(9):513–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

