Early-life iron deficiency persistently disrupts affective behaviour in mice
- PMID: 37096819
- PMCID: PMC10132221
- DOI: 10.1080/07853890.2023.2191003
Early-life iron deficiency persistently disrupts affective behaviour in mice
Abstract
Background/objective: Iron deficiency (ID) is the most common nutrient deficiency, affecting two billion people worldwide, including about 30% of pregnant women. During gestation, the brain is particularly vulnerable to environmental insults, which can irrevocably impair critical developmental processes. Consequently, detrimental consequences of early-life ID for offspring brain structure and function have been described. Although early life ID has been associated with an increased long-term risk for several neuropsychiatric disorders, the effect on depressive disorders has remained unresolved.
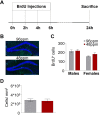
Materials and methods: A mouse model of moderate foetal and neonatal ID was established by keeping pregnant dams on an iron-deficient diet throughout gestation until postnatal day 10. The ensuing significant decrease of iron content in the offspring brain, as well as the impact on maternal behaviour and offspring vocalization was determined in the first postnatal week. The consequences of early-life ID for depression- and anxiety-like behaviour in adulthood were revealed employing dedicated behavioural assays. miRNA sequencing of hippocampal tissue of offspring revealed specific miRNAs signatures accompanying the behavioural deficits of foetal and neonatal ID in the adult brain.
Results: Mothers receiving iron-deficient food during pregnancy and lactation exhibited significantly less licking and grooming behaviour, while active pup retrieval and pup ultrasonic vocalizations were unaltered. Adult offspring with a history of foetal and neonatal ID showed an increase in depression- and anxiety-like behaviour, paralleled by a deranged miRNA expression profile in the hippocampus, specifically levels of miR200a and miR200b.
Conclusion: ID during the foetal and neonatal periods has life-long consequences for affective behaviour in mice and leaves a specific and persistent mark on the expression of miRNAs in the brain. Foetal and neonatal ID needs to be further considered as risk factor for the development of depression and anxiety disorders later in life.Key MessagesMarginal reduction of gestational alimentary iron intake decreases brain iron content of the juvenile offspring.Early-life ID is associated with increased depression- and anxiety-like behaviour in adulthood.Reduction of maternal alimentary iron intake during pregnancy is reflected in an alteration of miRNA signatures in the adult offspring brain.
Keywords: Iron deficiency; anxiety disorder; depression; hippocampus; miRNA profile; mouse behaviour.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Prenatal Choline Supplementation Diminishes Early-Life Iron Deficiency-Induced Reprogramming of Molecular Networks Associated with Behavioral Abnormalities in the Adult Rat Hippocampus.J Nutr. 2016 Mar;146(3):484-93. doi: 10.3945/jn.115.227561. Epub 2016 Feb 10. J Nutr. 2016. PMID: 26865644 Free PMC article.
-
Maternal iron deficiency alters essential fatty acid and eicosanoid metabolism and increases locomotion in adult guinea pig offspring.J Nutr. 2009 Sep;139(9):1653-9. doi: 10.3945/jn.109.106013. Epub 2009 Jul 29. J Nutr. 2009. PMID: 19640965
-
Additive effects of maternal iron deficiency and prenatal immune activation on adult behaviors in rat offspring.Brain Behav Immun. 2014 Aug;40:27-37. doi: 10.1016/j.bbi.2014.06.005. Epub 2014 Jun 12. Brain Behav Immun. 2014. PMID: 24930842
-
Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment.Hippocampus. 2012 Aug;22(8):1691-702. doi: 10.1002/hipo.22004. Epub 2012 Feb 27. Hippocampus. 2012. PMID: 22367974 Free PMC article.
-
The role of iron in learning and memory.Adv Nutr. 2011 Mar;2(2):112-21. doi: 10.3945/an.110.000190. Epub 2011 Mar 10. Adv Nutr. 2011. PMID: 22332040 Free PMC article. Review.
References
-
- Lozoff B, Beard J, Connor J, et al. . Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(5 Pt 2): s34–43. discussion S72-91. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1540447&tool=p... - PMC - PubMed
-
- Youdim MBH. Brain iron deficiency and excess; cognitive impairment and neurodegeneration with involvement of striatum and hippocampus. Neurotox Res. 2008;14(1):45–56. http://www.ncbi.nlm.nih.gov/pubmed/18790724 - PubMed
-
- Beard JL, Connor JR, Jones BC.. Iron in the brain. Nutr Rev. 1993;51(6):157–170. - PubMed
-
- Lozoff B, Brittenham GM, Wolf AW, et al. . Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics. 1987;79:981–995. http://www.ncbi.nlm.nih.gov/pubmed/2438638 - PubMed
-
- Rice D, Barone S.. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl):511–533. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1637807&tool=p... - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
