Terahertz Spectroscopy Sheds Light on Real-Time Exchange Kinetics Occurring through Plasma Membrane during Photodynamic Therapy Treatment
- PMID: 37096839
- PMCID: PMC10288265
- DOI: 10.1002/advs.202300589
Terahertz Spectroscopy Sheds Light on Real-Time Exchange Kinetics Occurring through Plasma Membrane during Photodynamic Therapy Treatment
Abstract
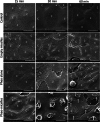
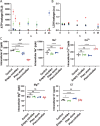
Methods to follow in real time complex processes occurring along living cell membranes such as cell permeabilization are rare. Here, the terahertz spectroscopy reveals early events in plasma membrane alteration generated during photodynamic therapy (PDT) protocol, events which are not observable in any other conventional biological techniques performed in parallel as comparison. Photodynamic process is examined in Madin-Darby canine kidney cells using Pheophorbide (Pheo) photosensitizer alone or alternatively encapsulated in poly(ethylene oxide)-block-poly(ε-caprolactone) micelles for drug delivery purpose. Terahertz spectroscopy (THz) reveals that plasma membrane permeabilization starts simultaneously with illumination and is stronger when photosensitizer is encapsulated. In parallel, the exchange of biological species is assessed. Over several hours, this conventional approach demonstrates significant differences between free and encapsulated Pheo, the latter leading to high penetration of propidium iodide, Na+ and Ca2+ ions, and a high level of leakage of K+ , ATP, and lactate dehydrogenase. THz spectroscopy provides, in a single measurement, the relative number of defects per membrane surface created after PDT, which is not achieved by any other method, providing early, sensitive real-time information. THz spectroscopy is therefore a promising technique and can be applied to any biological topic requiring the examination of short-term plasma membrane permeabilization.
Keywords: permeability; photodynamic therapy PDT; plasma membrane; polymers; self-assembly.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
 = empty micelles,
= empty micelles,  = Pheo alone,
= Pheo alone,  = Pheo‐micelles.
= Pheo‐micelles.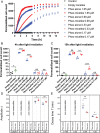
 = Pheo alone,
= Pheo alone,  = Pheo‐micelles.
= Pheo‐micelles.

References
-
- Smolyanskaya O. A., Chernomyrdin N. V., Konovko A. A., Zaytsev K. I., Ozheredov I. A., Cherkasova O. P., Nazarov M. M., Guillet J. P., Kozlov S. A., Kistenev Y. V., Coutaz J. L., Mounaix P., Vaks V. L., Son J. H., Cheon H., Wallace V. P., Feldman Y., Popov I., Yaroslaysky A. N., Shkurinov A. P., Tuchin V. V., Prog. Quantum Electron. 2018, 62, 1.
-
- Woodward R. M., Cole B. E., Wallace V. P., Pye R. J., Arnone D. D., Linfield E. H., Pepper M., Phys. Med. Biol. 2002, 47, 3853. - PubMed
-
- Masson J.‐B., Sauviat M.‐P., Gallot G., Appl. Phys. Lett. 2006, 89, 153904.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
