A new kid in the folding funnel: Molecular chaperone activities of the BRICHOS domain
- PMID: 37096906
- PMCID: PMC10182729
- DOI: 10.1002/pro.4645
A new kid in the folding funnel: Molecular chaperone activities of the BRICHOS domain
Abstract
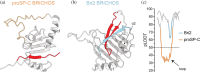
The BRICHOS protein superfamily is a diverse group of proteins associated with a wide variety of human diseases, including respiratory distress, COVID-19, dementia, and cancer. A key characteristic of these proteins-besides their BRICHOS domain present in the ER lumen/extracellular part-is that they harbor an aggregation-prone region, which the BRICHOS domain is proposed to chaperone during biosynthesis. All so far studied BRICHOS domains modulate the aggregation pathway of various amyloid-forming substrates, but not all of them can keep denaturing proteins in a folding-competent state, in a similar manner as small heat shock proteins. Current evidence suggests that the ability to interfere with the aggregation pathways of substrates with entirely different end-point structures is dictated by BRICHOS quaternary structure as well as specific surface motifs. This review aims to provide an overview of the BRICHOS protein family and a perspective of the diverse molecular chaperone-like functions of various BRICHOS domains in relation to their structure and conformational plasticity. Furthermore, we speculate about the physiological implication of the diverse molecular chaperone functions and discuss the possibility to use the BRICHOS domain as a blood-brain barrier permeable molecular chaperone treatment of protein aggregation disorders.
Keywords: Alzheimer disease treatment; amyloid; blood-brain barrier; molecular chaperone; protein misfolding.
© 2023 The Authors. Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Figures



Similar articles
-
Folding and Intramembraneous BRICHOS Binding of the Prosurfactant Protein C Transmembrane Segment.J Biol Chem. 2015 Jul 10;290(28):17628-41. doi: 10.1074/jbc.M114.630343. Epub 2015 Jun 3. J Biol Chem. 2015. PMID: 26041777 Free PMC article.
-
Short hydrophobic loop motifs in BRICHOS domains determine chaperone activity against amorphous protein aggregation but not against amyloid formation.Commun Biol. 2023 May 8;6(1):497. doi: 10.1038/s42003-023-04883-2. Commun Biol. 2023. PMID: 37156997 Free PMC article.
-
BRICHOS domain associated with lung fibrosis, dementia and cancer--a chaperone that prevents amyloid fibril formation?FEBS J. 2011 Oct;278(20):3893-904. doi: 10.1111/j.1742-4658.2011.08209.x. Epub 2011 Jul 5. FEBS J. 2011. PMID: 21668643 Review.
-
Molecular basis for different substrate-binding sites and chaperone functions of the BRICHOS domain.Protein Sci. 2024 Jul;33(7):e5063. doi: 10.1002/pro.5063. Protein Sci. 2024. PMID: 38864729 Free PMC article.
-
Updates on Aβ Processing by Hsp90, BRICHOS, and Newly Reported Distinctive Chaperones.Biomolecules. 2023 Dec 22;14(1):16. doi: 10.3390/biom14010016. Biomolecules. 2023. PMID: 38254616 Free PMC article. Review.
Cited by
-
Metal Binding of Alzheimer's Amyloid-β and Its Effect on Peptide Self-Assembly.Acc Chem Res. 2023 Oct 3;56(19):2653-2663. doi: 10.1021/acs.accounts.3c00370. Epub 2023 Sep 21. Acc Chem Res. 2023. PMID: 37733746 Free PMC article.
-
Protein folding: Funnel model revised.Comput Struct Biotechnol J. 2024 Oct 21;23:3827-3838. doi: 10.1016/j.csbj.2024.10.030. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39525086 Free PMC article.
-
Potential of molecular chaperones for treating Alzheimer's disease.Neural Regen Res. 2024 Nov 1;19(11):2343-2344. doi: 10.4103/NRR.NRR-D-23-01927. Epub 2024 Mar 8. Neural Regen Res. 2024. PMID: 38526266 Free PMC article. No abstract available.
-
Myelin Basic Protein Attenuates Furin-Mediated Bri2 Cleavage and Postpones Its Membrane Trafficking.Int J Mol Sci. 2024 Feb 23;25(5):2608. doi: 10.3390/ijms25052608. Int J Mol Sci. 2024. PMID: 38473856 Free PMC article.
-
Intravenous chaperone treatment of late-stage Alzheimer´s disease (AD) mouse model affects amyloid plaque load, reactive gliosis and AD-related genes.Transl Psychiatry. 2024 Oct 24;14(1):453. doi: 10.1038/s41398-024-03161-x. Transl Psychiatry. 2024. PMID: 39448576 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

