In Search of Wasserman's Catenane
- PMID: 37096971
- PMCID: PMC10161206
- DOI: 10.1021/jacs.3c01939
In Search of Wasserman's Catenane
Abstract
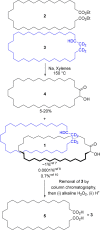
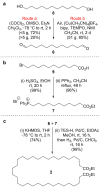
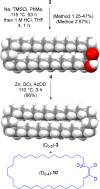
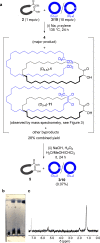
We repeat the earliest claimed [2]catenane synthesis, reported by Wasserman over 60 years ago, in order to ascertain whether or not a nontemplate, statistical synthesis by acyloin macrocyclization does indeed form mechanically interlocked rings. The lack of direct experimental evidence for Wasserman's catenane has led to it being described as a "prophetic compound", a technical term used in patents for claimed molecules that have not yet been synthesized. Contemporary synthetic methods were used to reconstruct Wasserman's deuterium-labeled macrocycle and other building blocks on the 10-100 g reaction scale necessary to generate, in principle, ∼1 mg of catenane. Modern spectrometric and spectroscopic tools and chemical techniques (including tandem mass spectrometry, deuterium nuclear magnetic resonance (NMR) spectroscopy, and fluorescent tag labeling) were brought to bear in an effort to detect, isolate, and prove the structure of a putative [2]catenane consisting of a 34-membered cyclic hydrocarbon mechanically linked with a 34-membered cyclic α-hydroxyketone.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Frisch H. L.; Wasserman E. Chemical Topology. J. Am. Chem. Soc. 1961, 83, 3789–3795. 10.1021/ja01479a015. - DOI
-
- Sauvage J.-P., Dietrich-Buchecker C., Eds. Molecular Catenanes, Rotaxanes and Knots. A Journey Through the World of Molecular Topology; Wiley-VCH: Weinheim, 1999.
-
- Bruns C. J; Stoddart J. F.. The Nature of the Mechanical Bond: From Molecules to Machines; John Wiley & Sons: Hoboken, NJ, 2017.
LinkOut - more resources
Full Text Sources

