Cell Entry of Avian Reovirus Modulated by Cell-Surface Annexin A2 and Adhesion G Protein-Coupled Receptor Latrophilin-2 Triggers Src and p38 MAPK Signaling Enhancing Caveolin-1- and Dynamin 2-Dependent Endocytosis
- PMID: 37097149
- PMCID: PMC10269738
- DOI: 10.1128/spectrum.00009-23
Cell Entry of Avian Reovirus Modulated by Cell-Surface Annexin A2 and Adhesion G Protein-Coupled Receptor Latrophilin-2 Triggers Src and p38 MAPK Signaling Enhancing Caveolin-1- and Dynamin 2-Dependent Endocytosis
Abstract
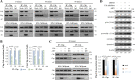
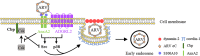
The specifics of cell receptor-modulated avian reovirus (ARV) entry remain unknown. By using a viral overlay protein-binding assay (VOPBA) and an in-gel digestion coupled with liquid chromatography-tandem mass spectrometry (LC-MS/MS), we determined that cell-surface annexin A2 (AnxA2) and adhesion G protein-coupled receptor Latrophilin-2 (ADGRL2) modulate ARV entry. Direct interaction between the ARV σC protein and AnxA2 and ADGRL2 in Vero and DF-1 cells was demonstrated in situ by proximity ligation assays. By using short hairpin RNAs (shRNAs) to silence the endogenous AnxA2 and ADGRL2 genes, ARV entry could be efficiently blocked. A significant decrease in virus yields and the intracellular specific signal for σC protein was observed in Vero cells preincubated with the specific AnxA2 and ADGRL2 monoclonal antibodies, indicating that AnxA2 and ADGRL2 are involved in modulating ARV entry. Furthermore, we found that cells pretreated with the AnxA2/S100A10 heterotetramer (A2t) inhibitor A2ti-1 suppressed ARV-mediated activation of Src and p38 mitogen-activated protein kinase (MAPK), demonstrating that Src and p38 MAPK serve as downstream molecules of cell-surface AnxA2 signaling. Our results reveal that suppression of cell-surface AnxA2 with the A2ti-1 inhibitor increased Csk-Cbp interaction, suggesting that ARV entry suppresses Cbp-mediated relocation of Csk to the membrane, thereby activating Src. Furthermore, reciprocal coimmunoprecipitation assays revealed that σC can interact with signaling molecules, lipid raft, and vimentin. The current study provides novel insights into cell-surface AnxA2- and ADGRL2-modulated cell entry of ARV which triggers Src and p38 MAPK signaling to enhance caveolin-1-, dynamin 2-, and lipid raft-dependent endocytosis. IMPORTANCE By analyzing results from VOPBA and LC-MS/MS, we have determined that cell-surface AnxA2 and ADGRL2 modulate ARV entry. After ARV binding to receptors, Src and p38 MAPK signaling were triggered and, in turn, increased the phosphorylation of caveolin-1 (Tyr14) and upregulated dynamin 2 expression to facilitate caveolin-1-mediated and dynamin 2-dependent endocytosis. In this work, we demonstrated that ARV triggers Src activation by impeding Cbp-mediated relocation of Csk to the membrane in the early stages of the life cycle. This work provides better insight into cell-surface AnxA2 and ADGRL2, which upregulate Src and p38MAPK signaling pathways to enhance ARV entry and productive infection.
Keywords: ADGRL2; Csk-Cbp interaction; Src; annexin A2; avian reovirus; caveolin-1; dynamin 2; p38 MAPK; σC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hofstad MS. 1978. Diseases of poultry. Iowa State University Press, Ames, Iowa.
-
- Kozak RA, Hattin L, Biondi MJ, Corredor JC, Walsh S, Xue-Zhong M, Manuel J, McGilvray ID, Morgenstern J, Lusty E, Cherepanov V, McBey BA, Leishman D, Feld JJ, Bridle B, Nagy É. 2017. Replication and oncolytic activity of an avian orthoreovirus in human hepatocellular carcinoma cells. Viruses 9:90. doi: 10.3390/v9040090. - DOI - PMC - PubMed
-
- Chiu HC, Huang WR, Liao TL, Chi PI, Nielsen BL, Liu JH, Liu HJ. 2018. Mechanistic insights into avian reovirus p17-modulated suppression of cell cycle CDK-cyclin complexes and enhancement of p53 and cyclin H interaction. J Biol Chem 293:12542–12562. doi: 10.1074/jbc.RA118.002341. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

