Hif-1α/Slit2 Mediates Vascular Smooth Muscle Cell Phenotypic Changes in Restenosis of Bypass Grafts
- PMID: 37097589
- PMCID: PMC10615989
- DOI: 10.1007/s12265-023-10384-8
Hif-1α/Slit2 Mediates Vascular Smooth Muscle Cell Phenotypic Changes in Restenosis of Bypass Grafts
Abstract
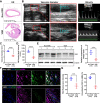
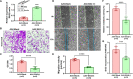
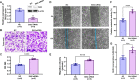
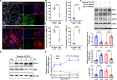
Vascular smooth muscle cells (VSMCs) are involved in restenosis of bypass grafts and cause artery graft occlusion. This study aimed to explore the role of Slit2 in phenotypic switching of VSMCs and its effect on restenosis of vascular conduits. An animal model of vascular graft restenosis (VGR) was produced in SD rats and assessed by echocardiography. The expression of Slit2 and Hif-1α was measured in vivo and in vitro. After Slit2 overexpression, the migration and proliferation of VSMCs were detected in vitro, and the restenosis rates and phenotype of VSMCs were tested in vivo. The arteries of the VGR model presented significant stenosis, and Slit2 was decreased in VSMCs of the VGR model. In vitro, Slit2 overexpression inhibited the migration and proliferation of VSMCs, but Slit2 knockdown promoted migration and proliferation. Hypoxia induced Hif-1α but reduced Slit2, and Hif-1α negatively regulated Slit2 expression. Moreover, Slit2 overexpression weakened the rate of VGR and maintained the patency of artery bypass grafts, which suppressed the phenotypic switching of VSMCs. Slit2 inhibited the synthetic phenotype transformation to inhibit the migration and proliferation of VSMCs and delayed the VGR via Hif-1α.
Keywords: Bypass graft; Hif-1α; Restenosis; Slit2; Smooth muscle cell.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

