Ultrasound Responsive Nanovaccine Armed with Engineered Cancer Cell Membrane and RNA to Prevent Foreseeable Metastasis
- PMID: 37097746
- PMCID: PMC10323640
- DOI: 10.1002/advs.202301107
Ultrasound Responsive Nanovaccine Armed with Engineered Cancer Cell Membrane and RNA to Prevent Foreseeable Metastasis
Abstract
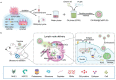
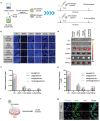
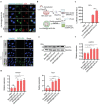
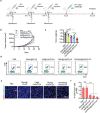
Cancer vaccine has been considered as a promising immunotherapy by inducing specific anti-tumor immune response. Rational vaccination at suitable time to efficiently present tumor associated antigen will boost tumor immunity and is badly needed. Here, a poly (lactic-co-glycolic acid) (PLGA)-based cancer vaccine of nanoscale is designed, in which engineered tumor cell membrane proteins, mRNAs, and sonosensitizer chlorin e6 (Ce6) are encapsulated at high efficiency. The nanosized vaccine can be efficiently delivered into antigen presentation cells (APCs) in lymph nodes after subcutaneous injection. In the APCs, the encapsulated cell membrane and RNA from engineered cells, which have disturbed splicing resembling the metastatic cells, provide neoantigens of metastatic cancer in advance. Moreover, the sonosensitizer Ce6 together with ultrasound irradiation promotes mRNA escape from endosome, and augments antigen presentation. Through 4T1 syngeneic mouse model, it has been proved that the proposed nanovaccine is efficient to elicit antitumor immunity and thus prevent cancer metastasis.
Keywords: alternative splicing; antigen presentation; cancer vaccine; sonosensitizer.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Immune stimulatory antigen loaded particles combined with depletion of regulatory T-cells induce potent tumor specific immunity in a mouse model of melanoma.Cancer Immunol Immunother. 2009 Apr;58(4):517-30. doi: 10.1007/s00262-008-0574-6. Epub 2008 Aug 22. Cancer Immunol Immunother. 2009. PMID: 18719913 Free PMC article.
-
Erythrocyte Membrane-Enveloped Polymeric Nanoparticles as Nanovaccine for Induction of Antitumor Immunity against Melanoma.ACS Nano. 2015 Jul 28;9(7):6918-33. doi: 10.1021/acsnano.5b01042. Epub 2015 Jul 14. ACS Nano. 2015. PMID: 26153897
-
Tumor growth inhibition by mSTEAP peptide nanovaccine inducing augmented CD8+ T cell immune responses.Drug Deliv Transl Res. 2019 Dec;9(6):1095-1105. doi: 10.1007/s13346-019-00652-z. Drug Deliv Transl Res. 2019. PMID: 31228097
-
Harnessing Dendritic Cells for Poly (D,L-lactide-co-glycolide) Microspheres (PLGA MS)-Mediated Anti-tumor Therapy.Front Immunol. 2019 Apr 5;10:707. doi: 10.3389/fimmu.2019.00707. eCollection 2019. Front Immunol. 2019. PMID: 31024545 Free PMC article. Review.
-
Efficient Nanovaccine Delivery in Cancer Immunotherapy.ACS Nano. 2017 Mar 28;11(3):2387-2392. doi: 10.1021/acsnano.7b00978. Epub 2017 Mar 9. ACS Nano. 2017. PMID: 28277646 Review.
Cited by
-
Cell Membrane-Camouflaged Nanoparticles Mediated Nucleic Acids Delivery.Int J Nanomedicine. 2023 Dec 28;18:8001-8021. doi: 10.2147/IJN.S433737. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38164266 Free PMC article. Review.
-
Smart Nanoarchitectures for Precision RNA Delivery: Harnessing Endogenous and Exogenous Stimuli in Cancer Treatment.Theranostics. 2025 Jul 2;15(15):7747-7778. doi: 10.7150/thno.112492. eCollection 2025. Theranostics. 2025. PMID: 40756358 Free PMC article. Review.
-
Tumor cell membrane-based vaccines: A potential boost for cancer immunotherapy.Exploration (Beijing). 2024 Mar 28;4(6):20230171. doi: 10.1002/EXP.20230171. eCollection 2024 Dec. Exploration (Beijing). 2024. PMID: 39713208 Free PMC article. Review.
-
Ultrasound combined with microbubble mediated immunotherapy for tumor microenvironment.Front Pharmacol. 2024 Feb 29;15:1304502. doi: 10.3389/fphar.2024.1304502. eCollection 2024. Front Pharmacol. 2024. PMID: 38487163 Free PMC article. Review.
-
Nanomaterial Delivery Vehicles for the Development of Neoantigen Tumor Vaccines for Personalized Treatment.Molecules. 2024 Mar 25;29(7):1462. doi: 10.3390/molecules29071462. Molecules. 2024. PMID: 38611742 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
