First-in-class multifunctional TYMS nonclassical antifolate inhibitor with potent in vivo activity that prolongs survival
- PMID: 37097751
- PMCID: PMC10386886
- DOI: 10.1172/jci.insight.158798
First-in-class multifunctional TYMS nonclassical antifolate inhibitor with potent in vivo activity that prolongs survival
Abstract
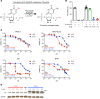
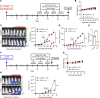
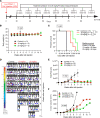
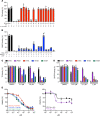
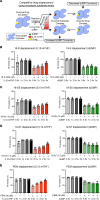
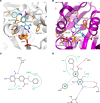
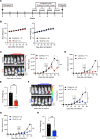
Although thymidylate synthase (TYMS) inhibitors have served as components of chemotherapy regimens, the currently available inhibitors induce TYMS overexpression or alter folate transport/metabolism feedback pathways that tumor cells exploit for drug resistance, limiting overall benefit. Here we report a small molecule TYMS inhibitor that i) exhibited enhanced antitumor activity as compared with current fluoropyrimidines and antifolates without inducing TYMS overexpression, ii) is structurally distinct from classical antifolates, iii) extended survival in both pancreatic xenograft tumor models and an hTS/Ink4a/Arf null genetically engineered mouse tumor model, and iv) is well tolerated with equal efficacy using either intraperitoneal or oral administration. Mechanistically, we verify the compound is a multifunctional nonclassical antifolate, and using a series of analogs, we identify structural features allowing direct TYMS inhibition while maintaining the ability to inhibit dihydrofolate reductase. Collectively, this work identifies nonclassical antifolate inhibitors that optimize inhibition of thymidylate biosynthesis with a favorable safety profile, highlighting the potential for enhanced cancer therapy.
Keywords: Cancer; Oncogenes; Therapeutics.
Figures


References
-
- Longley DB, et al. Characterization of a thymidylate synthase (TS)-inducible cell line: a model system for studying sensitivity to TS- and non-TS-targeted chemotherapies. Clin Cancer Res. 2001;7(11):3533–3539. - PubMed
-
- Johnston PG, et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995;55(7):1407–1412. - PubMed
-
- Lenz HJ, et al. p53 and thymidylate synthase expression in untreated stage II colon cancer: associations with recurrence, survival, and site. Clin Cancer Res. 1998;4(5):1227–1234. - PubMed
-
- Salonga D, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6(4):1322–1327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

