MOGAD patient autoantibodies induce complement, phagocytosis, and cellular cytotoxicity
- PMID: 37097758
- PMCID: PMC10393237
- DOI: 10.1172/jci.insight.165373
MOGAD patient autoantibodies induce complement, phagocytosis, and cellular cytotoxicity
Abstract
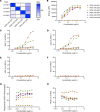
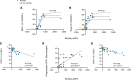
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is an inflammatory demyelinating CNS condition characterized by the presence of MOG autoantibodies. We sought to investigate whether human MOG autoantibodies are capable of mediating damage to MOG-expressing cells through multiple mechanisms. We developed high-throughput assays to measure complement activity (CA), complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent cellular cytotoxicity (ADCC) of live MOG-expressing cells. MOGAD patient sera effectively mediate all of these effector functions. Our collective analyses reveal that (a) cytotoxicity is not incumbent on MOG autoantibody quantity alone; (b) engagement of effector functions by MOGAD patient serum is bimodal, with some sera exhibiting cytotoxic capacity while others did not; (c) the magnitude of CDC and ADCP is elevated closer to relapse, while MOG-IgG binding is not; and (d) all IgG subclasses can damage MOG-expressing cells. Histopathology from a representative MOGAD case revealed congruence between lesion histology and serum CDC and ADCP, and we identified NK cells, mediators of ADCC, in the cerebrospinal fluid of relapsing patients with MOGAD. Thus, MOGAD-derived autoantibodies are cytotoxic to MOG-expressing cells through multiple mechanisms, and assays quantifying CDC and ADCP may prove to be effective tools for predicting risk of future relapses.
Keywords: Autoimmune diseases; Autoimmunity; Demyelinating disorders; Neurological disorders; Neuroscience.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

