Somatostatin regulates central clock function and circadian responses to light
- PMID: 37098068
- PMCID: PMC10160998
- DOI: 10.1073/pnas.2216820120
Somatostatin regulates central clock function and circadian responses to light
Abstract
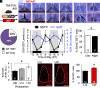
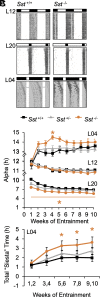
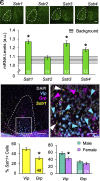
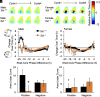
Daily and annual changes in light are processed by central clock circuits that control the timing of behavior and physiology. The suprachiasmatic nucleus (SCN) in the anterior hypothalamus processes daily photic inputs and encodes changes in day length (i.e., photoperiod), but the SCN circuits that regulate circadian and photoperiodic responses to light remain unclear. Somatostatin (SST) expression in the hypothalamus is modulated by photoperiod, but the role of SST in SCN responses to light has not been examined. Our results indicate that SST signaling regulates daily rhythms in behavior and SCN function in a manner influenced by sex. First, we use cell-fate mapping to provide evidence that SST in the SCN is regulated by light via de novo Sst activation. Next, we demonstrate that Sst -/- mice display enhanced circadian responses to light, with increased behavioral plasticity to photoperiod, jetlag, and constant light conditions. Notably, lack of Sst -/- eliminated sex differences in photic responses due to increased plasticity in males, suggesting that SST interacts with clock circuits that process light differently in each sex. Sst -/- mice also displayed an increase in the number of retinorecipient neurons in the SCN core, which express a type of SST receptor capable of resetting the molecular clock. Last, we show that lack of SST signaling modulates central clock function by influencing SCN photoperiodic encoding, network after-effects, and intercellular synchrony in a sex-specific manner. Collectively, these results provide insight into peptide signaling mechanisms that regulate central clock function and its response to light.
Keywords: behavior; circadian; photoperiod; somatostatin; suprachiasmatic nucleus.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Evans J. A., Davidson A. J., Health consequences of circadian disruption in humans and animal models. Prog. Mol. Biol. Transl. Sci. 119, 283–323 (2013). - PubMed
-
- McMenamin T. M., A time to work: Recent trends in shift work and flexible schedules. Mon. Labor. Rev. 130, 9–11 (2007).
-
- Wirz-Justice A., Seasonality in affective disorders. Gen. Comp. Endocrinol. 258, 244–249 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

