Rhinovirus C15 Attenuates Relaxation and cAMP Production in Human Airways and Smooth Muscle
- PMID: 37098126
- PMCID: PMC10399146
- DOI: 10.1165/rcmb.2021-0526OC
Rhinovirus C15 Attenuates Relaxation and cAMP Production in Human Airways and Smooth Muscle
Abstract
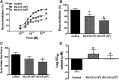
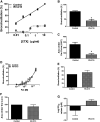
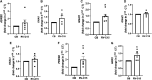
Rhinoviruses (RVs) evoke as many as 85% of acute asthma exacerbations in children and 50% in adults and can induce airway hyperresponsiveness and decrease efficacy of current therapeutics to provide symptom relief. Using human precision-cut lung slices (hPCLSs), primary human air-liquid interface-differentiated airway epithelial cells (HAECs), and human airway smooth muscle (HASM) as preclinical experimental models, we demonstrated that RV-C15 attenuates agonist-induced bronchodilation. Specifically, airway relaxation to formoterol and cholera toxin, but not forskolin (Fsk), was attenuated following hPCLS exposure to RV-C15. In isolated HASM cells, exposure to conditioned media from RV-exposed HAECs decreased cellular relaxation in response to isoproterenol and prostaglandin E2, but not Fsk. Additionally, cAMP generation elicited by formoterol and isoproterenol, but not Fsk, was attenuated following HASM exposure to RV-C15-conditioned HAEC media. HASM exposure to RV-C15-conditioned HAEC media modulated expression of components of relaxation pathways, specifically GNAI1 and GRK2. Strikingly, similar to exposure to intact RV-C15, hPCLS exposed to UV-inactivated RV-C15 showed markedly attenuated airway relaxation in response to formoterol, suggesting that the mechanism(s) of RV-C15-mediated loss of bronchodilation is independent of virus replication pathways. Further studies are warranted to identify soluble factor(s) regulating the epithelial-driven smooth muscle loss of β2-adrenergic receptor function.
Keywords: asthma; bronchodilation; exacerbations; rhinovirus.
Figures


Comment in
-
Remember the Airway Smooth Muscle! How Rhinovirus Impairs Bronchodilator Responses.Am J Respir Cell Mol Biol. 2023 Aug;69(2):121-122. doi: 10.1165/rcmb.2023-0146ED. Am J Respir Cell Mol Biol. 2023. PMID: 37163760 Free PMC article. No abstract available.
References
-
- Reddel H, Ware S, Marks G, Salome C, Jenkins C, Woolcock A. Differences between asthma exacerbations and poor asthma control. Lancet . 1999;353:364–369. - PubMed
-
- Reddel HK, Jenkins C, Quirce S, Sears MR, Bateman ED, O’Byrne PM, et al. Effect of different asthma treatments on risk of cold-related exacerbations. Eur Respir J . 2011;38:584–593. - PubMed
-
- Rueter K, Bizzintino J, Martin AC, Zhang G, Hayden CM, Geelhoed GC, et al. Symptomatic viral infection is associated with impaired response to treatment in children with acute asthma. J Pediatr . 2012;160:82–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

