Long-Term Outcomes and Molecular Correlates of Sotorasib Efficacy in Patients With Pretreated KRAS G12C-Mutated Non-Small-Cell Lung Cancer: 2-Year Analysis of CodeBreaK 100
- PMID: 37098232
- PMCID: PMC10414711
- DOI: 10.1200/JCO.22.02524
Long-Term Outcomes and Molecular Correlates of Sotorasib Efficacy in Patients With Pretreated KRAS G12C-Mutated Non-Small-Cell Lung Cancer: 2-Year Analysis of CodeBreaK 100
Abstract
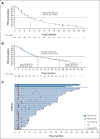
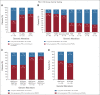
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.In the longest follow-up, to our knowledge, for a KRASG12C inhibitor, we assessed the long-term efficacy, safety, and biomarkers of sotorasib in patients with KRAS G12C-mutated advanced non-small-cell lung cancer (NSCLC) from the CodeBreaK 100 clinical trial (ClinicalTrials.gov identifier: NCT03600883). This multicenter, single-group, open-label phase I/phase II trial enrolled 174 patients with KRAS G12C-mutated, locally advanced or metastatic NSCLC after progression on prior therapies. Patients (N = 174) received sotorasib 960 mg once daily with the primary end points for phase I of safety and tolerability and for phase II of objective response rate (ORR). Sotorasib produced an ORR of 41%, median duration of response of 12.3 months, progression-free survival (PFS) of 6.3 months, overall survival (OS) of 12.5 months, and 2-year OS rate of 33%. Long-term clinical benefit (PFS ≥ 12 months) was observed in 40 (23%) patients across PD-L1 expression levels, in a proportion of patients with somatic STK11 and/or KEAP1 alterations, and was associated with lower baseline circulating tumor DNA levels. Sotorasib was well tolerated, with few late-onset treatment-related toxicities, none of which led to treatment discontinuation. These results demonstrate the long-term benefit of sotorasib, including in subgroups with poor prognosis.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Herbst RS, Baas P, Kim DW, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

