The Clinicogenomic Landscape of Induction Failure in Childhood and Young Adult T-Cell Acute Lymphoblastic Leukemia
- PMID: 37098241
- PMCID: PMC10306434
- DOI: 10.1200/JCO.22.02734
The Clinicogenomic Landscape of Induction Failure in Childhood and Young Adult T-Cell Acute Lymphoblastic Leukemia
Abstract
Purpose: Failure to respond to induction chemotherapy portends a poor outcome in childhood acute lymphoblastic leukemia (ALL) and is more frequent in T-cell ALL (T-ALL) than B-cell ALL. We aimed to address the limited understanding of clinical and genetic factors that influence outcome in a cohort of patients with T-ALL induction failure (IF).
Methods: We studied all cases of T-ALL IF on two consecutive multinational randomized trials, UKALL2003 and UKALL2011, to define risk factors, treatment, and outcomes. We performed multiomic profiling to characterize the genomic landscape.
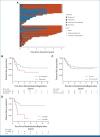
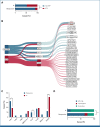
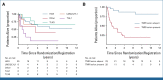
Results: IF occurred in 10.3% of cases and was significantly associated with increasing age, occurring in 20% of patients age 16 years and older. Five-year overall survival (OS) rates were 52.1% in IF and 90.2% in responsive patients (P < .001). Despite increased use of nelarabine-based chemotherapy consolidated by hematopoietic stem-cell transplant in UKALL2011, there was no improvement in outcome. Persistent end-of-consolidation molecular residual disease resulted in a significantly worse outcome (5-year OS, 14.3% v 68.5%; HR, 4.10; 95% CI, 1.35 to 12.45; P = .0071). Genomic profiling revealed a heterogeneous picture with 25 different initiating lesions converging on 10 subtype-defining genes. There was a remarkable abundance of TAL1 noncoding lesions, associated with a dismal outcome (5-year OS, 12.5%). Combining TAL1 lesions with mutations in the MYC and RAS pathways produces a genetic stratifier that identifies patients highly likely to fail conventional therapy (5-year OS, 23.1% v 86.4%; HR, 6.84; 95% CI, 2.78 to 16.78; P < .0001) and who should therefore be considered for experimental agents.
Conclusion: The outcome of IF in T-ALL remains poor with current therapy. The lack of a unifying genetic driver suggests alternative approaches, particularly using immunotherapy, are urgently needed.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- Hunger SP, Mullighan CG: Acute lymphoblastic leukemia in children. N Engl J Med 373:1541-1552, 2015 - PubMed
-
- O’Connor D, Moorman AV, Wade R, et al. : Use of minimal residual disease assessment to redefine induction failure in pediatric acute lymphoblastic leukemia. J Clin Oncol 35:660-667, 2017 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

