A tissue injury sensing and repair pathway distinct from host pathogen defense
- PMID: 37098344
- PMCID: PMC10321318
- DOI: 10.1016/j.cell.2023.03.031
A tissue injury sensing and repair pathway distinct from host pathogen defense
Abstract
Pathogen infection and tissue injury are universal insults that disrupt homeostasis. Innate immunity senses microbial infections and induces cytokines/chemokines to activate resistance mechanisms. Here, we show that, in contrast to most pathogen-induced cytokines, interleukin-24 (IL-24) is predominately induced by barrier epithelial progenitors after tissue injury and is independent of microbiome or adaptive immunity. Moreover, Il24 ablation in mice impedes not only epidermal proliferation and re-epithelialization but also capillary and fibroblast regeneration within the dermal wound bed. Conversely, ectopic IL-24 induction in the homeostatic epidermis triggers global epithelial-mesenchymal tissue repair responses. Mechanistically, Il24 expression depends upon both epithelial IL24-receptor/STAT3 signaling and hypoxia-stabilized HIF1α, which converge following injury to trigger autocrine and paracrine signaling involving IL-24-mediated receptor signaling and metabolic regulation. Thus, parallel to innate immune sensing of pathogens to resolve infections, epithelial stem cells sense injury signals to orchestrate IL-24-mediated tissue repair.
Keywords: STAT3; angiogenesis; coordinated tissue repair; epithelial stem cells; hypoxia; innate immune signaling; interferons; interleukin-24; microbiome-independent responses; tissue injury.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.L. is now an Asst. Prof. in Pharmacology at UT Southwestern Medical Center; X.C. is now an Asst. Prof. in Radiation Oncology at UT Southwestern Medical Center; K.A.U.G. is currently at Novo Nordisk, Research Center, Oxford, England; C.J.C. is now a postdoctoral fellow at NYU; T.T. is now a graduate student at Yale Univ.; B.H. is now a medical student at Weill Cornell Medical College; S.G.-C. is now an Asst. Prof. in Stem Cells and Regenerative Medicine at UCSD; M.S. is now an embryologist at Tennessee Reproductive Medicine in Chattanooga, TN; J.L. is currently at Temple Univ. C.B.T is a founder of Agios Pharmaceuticals. He is on the board of directors of Regeneron and Charles River Laboratories. E.F. is a member of the editorial board of Cell. She is also a former member of the scientific advisory boards of L’Oréal and Arsenal Biosciences and owns stock futures with Arsenal Biosciences.
Figures
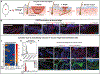
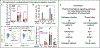





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

