Selectivity of osilodrostat as an inhibitor of human steroidogenic cytochromes P450
- PMID: 37098354
- PMCID: PMC10757358
- DOI: 10.1016/j.jsbmb.2023.106316
Selectivity of osilodrostat as an inhibitor of human steroidogenic cytochromes P450
Abstract
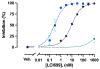
Osilodrostat (LCI699) is a potent inhibitor of the human steroidogenic cytochromes P450 11β-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2). LCI699 is FDA-approved for the treatment of Cushing disease, which is characterized by chronic overproduction of cortisol. While phase II and III clinical studies have proven the clinical efficacy and tolerability of LCI699 for treating Cushing disease, few studies have attempted to fully assess the effects of LCI699 on adrenal steroidogenesis. To this end, we first comprehensively analyzed LCI699-mediated inhibition of steroid synthesis in the NCI-H295R human adrenocortical cancer cell line. We then studied LCI699 inhibition using HEK-293 or V79 cells stably expressing individual human steroidogenic P450 enzymes. Our studies using intact cells confirm the potent inhibition of CYP11B1 and CYP11B2 with negligible inhibition of 17-hydroxylase/17,20-lyase (CYP17A1) and 21-hydroxylase (CYP21A2). Furthermore, partial inhibition of the cholesterol side-chain cleavage enzyme (CYP11A1) was observed. To calculate the dissociation constant (Kd) of LCI699 with the adrenal mitochondrial P450 enzymes, we successfully incorporated P450s into lipid nanodiscs and carried out spectrophotometric equilibrium and competition binding assays. Our binding experiments confirm the high affinity of LCI699 to CYP11B1 and CYP11B2 (Kd ≈ 1 nM or less) and much weaker binding for CYP11A1 (Kd = 18.8 μM). Our results confirm the selectivity of LCI699 for CYP11B1 and CYP11B2 and demonstrate partial inhibition of CYP11A1 but not CYP17A1 and CYP21A2.
Keywords: Cushing syndrome; Cytochrome P450; LCI699; Osilodrostat; Steroidogenesis.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interest RJA has received consulting fees from Recordati Rare Diseases and Novartis Pharmaceuticals, contracted research support from Novartis Pharmaceuticals, and a sample of osilodrostat solid used for the experiments described in this manuscript.
Figures

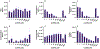

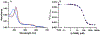

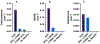
Similar articles
-
Functional maturation of the primate fetal adrenal in vivo: 3. Specific zonal localization and developmental regulation of CYP21A2 (P450c21) and CYP11B1/CYP11B2 (P450c11/aldosterone synthase) lead to integrated concept of zonal and temporal steroid biosynthesis.Endocrinology. 1998 Dec;139(12):5144-50. doi: 10.1210/endo.139.12.6333. Endocrinology. 1998. PMID: 9832454
-
1alpha,25-Dihydroxyvitamin D3 affects hormone production and expression of steroidogenic enzymes in human adrenocortical NCI-H295R cells.Biochim Biophys Acta. 2010 Sep;1801(9):1056-62. doi: 10.1016/j.bbalip.2010.04.009. Epub 2010 Apr 24. Biochim Biophys Acta. 2010. PMID: 20420936
-
Aldosterone synthase inhibition in humans.Nephrol Dial Transplant. 2013 Jan;28(1):36-43. doi: 10.1093/ndt/gfs388. Epub 2012 Oct 8. Nephrol Dial Transplant. 2013. PMID: 23045428 Review.
-
Osilodrostat Is a Potential Novel Steroidogenesis Inhibitor for the Treatment of Cushing Syndrome: An In Vitro Study.J Clin Endocrinol Metab. 2019 Aug 1;104(8):3437-3449. doi: 10.1210/jc.2019-00217. J Clin Endocrinol Metab. 2019. PMID: 31127821
-
The potential of targeting CYP11B.Expert Opin Ther Targets. 2016 Aug;20(8):923-34. doi: 10.1517/14728222.2016.1151873. Epub 2016 Mar 2. Expert Opin Ther Targets. 2016. PMID: 26854589 Review.
Cited by
-
Prediction of pharmacokinetic/pharmacodynamic properties of aldosterone synthase inhibitors at drug discovery stage using an artificial intelligence-physiologically based pharmacokinetic model.Front Pharmacol. 2025 Apr 28;16:1578117. doi: 10.3389/fphar.2025.1578117. eCollection 2025. Front Pharmacol. 2025. PMID: 40356995 Free PMC article.
-
Aldosterone Synthase Inhibitors for Cardiorenal Protection: Ready for Prime Time?Kidney Blood Press Res. 2024;49(1):1041-1056. doi: 10.1159/000542621. Epub 2024 Nov 18. Kidney Blood Press Res. 2024. PMID: 39557029 Free PMC article. Review.
-
Adrenodoxin allosterically alters human cytochrome P450 11B enzymes to accelerate substrate binding and decelerate release.RSC Chem Biol. 2024 Aug 2;5(9):938-51. doi: 10.1039/d4cb00015c. Online ahead of print. RSC Chem Biol. 2024. PMID: 39129792 Free PMC article.
-
Medical Treatment of Cushing's Syndrome.Endocrinol Metab (Seoul). 2025 Feb;40(1):26-38. doi: 10.3803/EnM.2024.501. Epub 2025 Jan 13. Endocrinol Metab (Seoul). 2025. PMID: 39801039 Free PMC article. Review.
-
Kinetics of Intermediate Release Enhances P450 11B2-Catalyzed Aldosterone Synthesis.Biochemistry. 2024 Apr 16;63(8):1026-1037. doi: 10.1021/acs.biochem.3c00725. Epub 2024 Apr 2. Biochemistry. 2024. PMID: 38564530 Free PMC article.
References
-
- Lacroix A, Feelders RA, Stratakis CA, Nieman LK, Cushing’s syndrome, Lancet, 386 (2015) 913–927. - PubMed
-
- Melmed S, Pituitary-Tumor Endocrinopathies, N Engl J Med, 382 (2020) 937–950. - PubMed
-
- Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, Shimatsu A, Gu F, Auchus R, Leelawattana R, Lee EJ, Kim JH, Lacroix A, Laplanche A, O’Connell P, Tauchmanova L, Pedroncelli AM, Biller BMK, investigators L, Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase, Lancet Diabetes Endocrinol, 8 (2020) 748–761. - PubMed
-
- Fleseriu M, Newell-Price J, Pivonello R, Shimatsu A, Auchus RJ, Scaroni C, Belaya Z, Feelders RA, Vila G, Houde G, Walia R, Izquierdo M, Roughton M, Pedroncelli AM, Biller BMK, Long-term outcomes of osilodrostat in Cushing’s disease: LINC 3 study extension, Eur J Endocrinol, 187 (2022) 531–541. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

