Rapid detection and diagnosis of herpetic keratitis using quantitative microfluidic polymerase chain reaction system for herpes simplex and varicella-zoster virus DNA: a case series
- PMID: 37098507
- PMCID: PMC10127024
- DOI: 10.1186/s12886-023-02938-w
Rapid detection and diagnosis of herpetic keratitis using quantitative microfluidic polymerase chain reaction system for herpes simplex and varicella-zoster virus DNA: a case series
Abstract
Background: A microfluidic real-time polymerase chain reaction (PCR) system can rapidly detect the viral DNA in specimens. Detection of herpes simplex virus (HSV) and varicella-zoster virus (VZV) DNA in tears is a useful diagnostic tool for herpes simplex virus keratitis (HSK) and herpes zoster ophthalmicus (HZO).
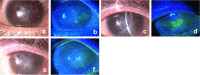
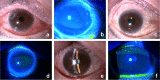
Methods: In total, 20 patients were included in this cross-sectional study. Among them, 8 patients with infectious epithelial HSK and 12 patients with HZO were included in HSK and HZO groups, respectively. In addition, 8 patients with non-herpetic keratitis and 4 healthy individuals without keratitis were included in the control group. Numbers of HSV and VZV DNA copies in tears of all patients and individuals were evaluated using a microfluidic real-time PCR system. Regarding HSV/VZV DNA test, tear specimens were collected by filter paper method using Schirmer's test paper, and subsequently, DNA was extracted from the filter paper using an automated nucleic acid extractor. Afterward, quantitative PCR was performed using a microfluidic real-time PCR system.
Results: From tear collection to real-time PCR result determination, the HSV/VZV DNA test took approximately 40 min. In the HSK group, the sensitivity and specificity of the HSV DNA tests were 100% each. The median value (range) of number of HSV DNA copies for affected eyes was 3.4 × 105 copies/μL (under a lower detection limit of 7.6). In the HZO group, the sensitivity and specificity of the VZV DNA tests were 100% each. The median value (range) of number of VZV DNA copies for affected eyes was 5.3 × 105 copies/μL (under a lower detection limit of 5.6 × 10-2).
Conclusion: In conclusion, quantitative PCR for HSV and VZV DNA in tears using a microfluidic real-time PCR system is useful for diagnosing and monitoring HSK and HZO.
Keywords: Conjunctivitis; Herpes simplex virus; Keratitis; Quantitative polymerase chain reaction; Varicella-zoster virus.
© 2023. The Author(s).
Conflict of interest statement
J.S. has previously received honoraria from Santen Pharmaceutical Co., Ltd., Senju Pharmaceutical Co., Ltd., AbbVie GK, and Rohto Nitten Co., Ltd., outside the submitted work. H.A., Y.S., N.I., Y.T., and R.A. declare that they have no conflicts of interest.
Figures


Similar articles
-
Evaluation of multiplex real-time polymerase chain reaction for the detection of herpes simplex virus-1 and 2 and varicella-zoster virus in corneal cells from normal subjects and patients with keratitis in India.Indian J Ophthalmol. 2019 Jul;67(7):1040-1046. doi: 10.4103/ijo.IJO_1700_18. Indian J Ophthalmol. 2019. PMID: 31238404 Free PMC article.
-
Detection of herpes simplex virus type 1, 2 and varicella zoster virus DNA in recipient corneal buttons.Br J Ophthalmol. 2000 Nov;84(11):1238-43. doi: 10.1136/bjo.84.11.1238. Br J Ophthalmol. 2000. PMID: 11049947 Free PMC article.
-
A diagnostic method for herpes simplex keratitis by simultaneous measurement of viral DNA and virus-specific secretory IgA in tears: an evaluation.Jpn J Ophthalmol. 2016 Jul;60(4):294-301. doi: 10.1007/s10384-016-0448-y. Epub 2016 Apr 28. Jpn J Ophthalmol. 2016. PMID: 27126382
-
Herpetic Eye Disease Following the SARS-CoV-2 Vaccinations.Ocul Immunol Inflamm. 2023 Aug;31(6):1151-1162. doi: 10.1080/09273948.2022.2103831. Epub 2022 Aug 1. Ocul Immunol Inflamm. 2023. PMID: 35914308 Review.
-
[Herpes simplex virus latency, reactivation, and a new antiviral therapy for herpetic keratitis].Nippon Ganka Gakkai Zasshi. 2008 Mar;112(3):247-64; discussion 265. Nippon Ganka Gakkai Zasshi. 2008. PMID: 18411713 Review. Japanese.
Cited by
-
Herpes simplex keratitis: A brief clinical overview.World J Virol. 2024 Mar 25;13(1):89934. doi: 10.5501/wjv.v13.i1.89934. World J Virol. 2024. PMID: 38616855 Free PMC article. Review.
References
-
- Shoji J, Sakimoto T, Inada N, Kamei Y, Matsubara M, Takamura E, et al. A diagnostic method for herpes simplex keratitis by simultaneous measurement of viral DNA and virus-specific secretory IgA in tears: an evaluation. Jpn J Ophthalmol. 2016;60:294–301. doi: 10.1007/s10384-016-0448-y. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

