Sodium selenite preserves rBM-MSCs' stemness, differentiation potential, and immunophenotype and protects them against oxidative stress via activation of the Nrf2 signaling pathway
- PMID: 37098557
- PMCID: PMC10127330
- DOI: 10.1186/s12906-023-03952-7
Sodium selenite preserves rBM-MSCs' stemness, differentiation potential, and immunophenotype and protects them against oxidative stress via activation of the Nrf2 signaling pathway
Abstract
Background: The physiological level of reactive oxygen species (ROS) is necessary for many cellular functions. However, during the in-vitro manipulations, cells face a high level of ROS, leading to reduced cell quality. Preventing this abnormal ROS level is a challenging task. Hence, here we evaluated the effect of sodium selenite supplementation on the antioxidant potential, stemness capacity, and differentiation of rat-derived Bone Marrow MSCs (rBM-MSCs) and planned to check our hypothesis on the molecular pathways and networks linked to sodium selenite's antioxidant properties.
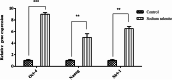
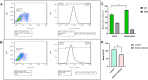
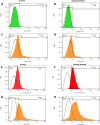
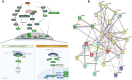
Methods: MTT assay was used to assess the rBM-MSCs cells' viability following sodium selenite supplementation (concentrations of: 0.001, 0.01, 0.1, 1, 10 µM). The expression level of OCT-4, NANOG, and SIRT1 was explored using qPCR. The adipocyte differentiation capacity of MSCs was checked after Sodium Selenite treatment. The DCFH-DA assay was used to determine intracellular ROS levels. Sodium selenite-related expression of HIF-1α, GPX, SOD, TrxR, p-AKT, Nrf2, and p38 markers was determined using western blot. Significant findings were investigated by the String tool to picture the probable molecular network.
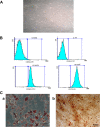
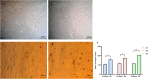
Results: Media supplemented with 0.1 µM sodium selenite helped to preserve rBM-MSCs multipotency and keep their surface markers presentation; this also reduced the ROS level and improved the rBM-MSCs' antioxidant and stemness capacity. We observed enhanced viability and reduced senescence for rBM-MSCs. Moreover, sodium selenite helped in rBM-MSCs cytoprotection by regulating the expression of HIF-1 of AKT, Nrf2, SOD, GPX, and TrxR markers.
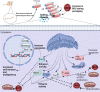
Conclusions: We showed that sodium selenite could help protect MSCs during in-vitro manipulations, probably via the Nrf2 pathway.
Keywords: Mesenchymal stem cells; Nrf pathway; Reactive oxygen species (ROS); Selenium selenite; Stemness.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
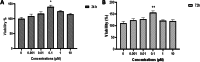

Similar articles
-
Mesenchymal Stem Cells Modified with Heme Oxygenase-1 Have Enhanced Paracrine Function and Attenuate Lipopolysaccharide-Induced Inflammatory and Oxidative Damage in Pulmonary Microvascular Endothelial Cells.Cell Physiol Biochem. 2018;49(1):101-122. doi: 10.1159/000492847. Epub 2018 Aug 28. Cell Physiol Biochem. 2018. PMID: 30153667
-
Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate experimental non-alcoholic steatohepatitis via Nrf2/NQO-1 pathway.Free Radic Biol Med. 2022 Nov 1;192:25-36. doi: 10.1016/j.freeradbiomed.2022.08.037. Epub 2022 Sep 10. Free Radic Biol Med. 2022. PMID: 36096356
-
Fucoxanthin diminishes oxidative stress damage in human placenta-derived mesenchymal stem cells through the PI3K/Akt/Nrf-2 pathway.Sci Rep. 2023 Dec 27;13(1):22974. doi: 10.1038/s41598-023-49751-5. Sci Rep. 2023. PMID: 38151503 Free PMC article.
-
Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway.Biol Trace Elem Res. 2012 Dec;150(1-3):441-50. doi: 10.1007/s12011-012-9488-4. Epub 2012 Aug 15. Biol Trace Elem Res. 2012. PMID: 22890880
-
Synergistic Antioxidant Effects of Molecular Hydrogen and Cold Atmospheric Plasma in Enhancing Mesenchymal Stem Cell Therapy.Antioxidants (Basel). 2024 Dec 23;13(12):1584. doi: 10.3390/antiox13121584. Antioxidants (Basel). 2024. PMID: 39765910 Free PMC article. Review.
Cited by
-
Selenium-modified hydroxyapatite titanium coating: enhancing osteogenesis and inhibiting cancer in bone invasion by head and neck squamous cell carcinoma.Front Bioeng Biotechnol. 2025 Feb 24;13:1552661. doi: 10.3389/fbioe.2025.1552661. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40066360 Free PMC article.
-
Selenium Nanoparticles Suppressed Oxidative Stress and Promoted Tenocyte Marker Expression in Tendon-Derived Stem/Progenitor Cells.Antioxidants (Basel). 2024 Dec 15;13(12):1536. doi: 10.3390/antiox13121536. Antioxidants (Basel). 2024. PMID: 39765864 Free PMC article.
-
Sodium Selenite Ameliorates Silver Nanoparticles Induced Vascular Endothelial Cytotoxic Injury by Antioxidative Properties and Suppressing Inflammation Through Activating the Nrf2 Signaling Pathway.Biol Trace Elem Res. 2024 Oct;202(10):4567-4585. doi: 10.1007/s12011-023-04014-2. Epub 2023 Dec 27. Biol Trace Elem Res. 2024. PMID: 38150116 Free PMC article.
-
Injectable thermosensitive selenium-containing hydrogel as mesenchymal stem cell carrier to improve treatment efficiency in limb ischemia.Mater Today Bio. 2024 Jan 20;25:100967. doi: 10.1016/j.mtbio.2024.100967. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38312804 Free PMC article.
References
-
- Khalili M, Zarebkohan A, Dianat-Moghadam H, Panahi M, Andre H, Alizadeh E. Corneal endothelial cell sheet bioengineering from neural crest cell-derived adipose stem cells on novel thermo-responsive elastin-mimetic dendrimers decorated with RGD. Chem Eng J. 2022;429:132523. doi: 10.1016/j.cej.2021.132523. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

