A Cross-Inoculation Experiment Reveals that Ophidiomyces ophiodiicola and Nannizziopsis guarroi Can Each Infect Both Snakes and Lizards
- PMID: 37098892
- PMCID: PMC10231240
- DOI: 10.1128/aem.02168-22
A Cross-Inoculation Experiment Reveals that Ophidiomyces ophiodiicola and Nannizziopsis guarroi Can Each Infect Both Snakes and Lizards
Abstract
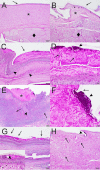
Host range and specificity are key concepts in the study of infectious diseases. However, both concepts remain largely undefined for many influential pathogens, including many fungi within the Onygenales order. This order encompasses reptile-infecting genera (Nannizziopsis, Ophidiomyces, and Paranannizziopsis) formerly classified as the Chrysosporium anamorph of Nannizziopsis vriesii (CANV). The reported hosts of many of these fungi represent a narrow range of phylogenetically related animals, suggesting that many of these disease-causing fungi are host specific, but the true number of species affected by these pathogens is unknown. For example, to date, Nannizziopsis guarroi (the causative agent of yellow fungus disease) and Ophidiomyces ophiodiicola (the causative agent of snake fungal disease) have been documented only in lizards and snakes, respectively. In a 52-day reciprocal-infection experiment, we tested the ability of these two pathogens to infect currently unreported hosts, inoculating central bearded dragons (Pogona vitticeps) with O. ophiodiicola and corn snakes (Pantherophis guttatus) with N. guarroi. We confirmed infection by documenting both clinical signs and histopathological evidence of fungal infection. Our reciprocity experiment resulted in 100% of corn snakes and 60% of bearded dragons developing infections with N. guarroi and O. ophiodiicola, respectively, demonstrating that these fungal pathogens have a broader host range than previously thought and that hosts with cryptic infections may play a role in pathogen translocation and transmission. IMPORTANCE Our experiment using Ophidiomyces ophiodiicola and Nannizziopsis guarroi is the first to look more critically at these pathogens' host range. We are the first to identify that both fungal pathogens can infect both corn snakes and bearded dragons. Our findings illustrate that both fungal pathogens have a more general host range than previously known. Additionally, there are significant implications concerning the spread of snake fungal disease and yellow fungus disease in popular companion animals and the increased chance of disease spillover into other wild and naive populations.
Keywords: CANV; Chrysosporium anamorph of Nannizziopsis vriesii; clinical experiment; emerging infectious disease; histopathology; host specificity; mycology; mycoses; snake fungal disease; spillover; yellow fungus disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Molecular characterization of reptile pathogens currently known as members of the chrysosporium anamorph of Nannizziopsis vriesii complex and relationship with some human-associated isolates.J Clin Microbiol. 2013 Oct;51(10):3338-57. doi: 10.1128/JCM.01465-13. Epub 2013 Aug 7. J Clin Microbiol. 2013. PMID: 23926168 Free PMC article.
-
Koch's postulates: Confirming Nannizziopsis guarroi as the cause of yellow fungal disease in Pogona vitticeps.Mycologia. 2021 Nov-Dec;113(6):1253-1263. doi: 10.1080/00275514.2021.1954445. Epub 2021 Sep 3. Mycologia. 2021. PMID: 34477498
-
Infection with Nannizziopsis guarroi and Ophidiomyces ophiodiicola in reptiles in Taiwan.Transbound Emerg Dis. 2022 Mar;69(2):764-775. doi: 10.1111/tbed.14049. Epub 2021 Mar 9. Transbound Emerg Dis. 2022. PMID: 33638294
-
Snake fungal disease: an emerging threat to wild snakes.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 5;371(1709):20150457. doi: 10.1098/rstb.2015.0457. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 28080983 Free PMC article. Review.
-
Chrysosporium anamorph Nannizziopsis vriesii: an emerging fungal pathogen of captive and wild reptiles.Vet Clin North Am Exot Anim Pract. 2013 Sep;16(3):659-68. doi: 10.1016/j.cvex.2013.05.013. Epub 2013 Jul 17. Vet Clin North Am Exot Anim Pract. 2013. PMID: 24018030 Review.
References
-
- Goater TM, Goater CP, Esch GW. 2001. Effects of parasites on their hosts: from individuals to ecosystems, p 396–427. In Halliday K, Waddington M (ed), Parasitism: the diversity and ecology of animal parasites, 2nd ed. Cambridge University Press, New York, NY.
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

