Adaptive Laboratory Evolution Reveals the Selenium Efflux Process To Improve Selenium Tolerance Mediated by the Membrane Sulfite Pump in Saccharomyces cerevisiae
- PMID: 37098949
- PMCID: PMC10269739
- DOI: 10.1128/spectrum.01326-23
Adaptive Laboratory Evolution Reveals the Selenium Efflux Process To Improve Selenium Tolerance Mediated by the Membrane Sulfite Pump in Saccharomyces cerevisiae
Abstract
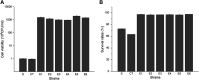
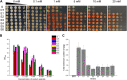
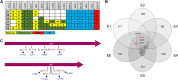
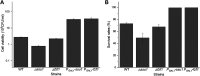
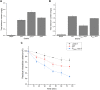
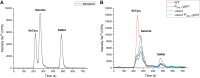
Selenium (Se) is a micronutrient in most eukaryotes, and Se-enriched yeast is the most common selenium supplement. However, selenium metabolism and transport in yeast have remained unclear, greatly hindering the application of this element. To explore the latent selenium transport and metabolism mechanisms, we performed adaptive laboratory evolution under the selective pressure of sodium selenite and successfully obtained selenium-tolerant yeast strains. Mutations in the sulfite transporter gene ssu1 and its transcription factor gene fzf1 were found to be responsible for the tolerance generated in the evolved strains, and the selenium efflux process mediated by ssu1 was identified in this study. Moreover, we found that selenite is a competitive substrate for sulfite during the efflux process mediated by ssu1, and the expression of ssu1 is induced by selenite rather than sulfite. Based on the deletion of ssu1, we increased the intracellular selenomethionine content in Se-enriched yeast. This work confirms the existence of the selenium efflux process, and our findings may benefit the optimization of Se-enriched yeast production in the future. IMPORTANCE Selenium is an essential micronutrient for mammals, and its deficiency severely threatens human health. Yeast is the model organism for studying the biological role of selenium, and Se-enriched yeast is the most popular selenium supplement to solve Se deficiency. The cognition of selenium accumulation in yeast always focuses on the reduction process. Little is known about selenium transport, especially selenium efflux, which may play a crucial part in selenium metabolism. The significance of our research is in determining the selenium efflux process in Saccharomyces cerevisiae, which will greatly enhance our knowledge of selenium tolerance and transport, facilitating the production of Se-enriched yeast. Moreover, our research further advances the understanding of the relationship between selenium and sulfur in transport.
Keywords: Se-enriched yeast; adaptive laboratory evolution; selenium; selenium efflux; selenium tolerance; yeasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




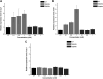
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

