Regulatory Role of Anti-Sigma Factor RsbW in Clostridioides difficile Stress Response, Persistence, and Infection
- PMID: 37098979
- PMCID: PMC10210984
- DOI: 10.1128/jb.00466-22
Regulatory Role of Anti-Sigma Factor RsbW in Clostridioides difficile Stress Response, Persistence, and Infection
Abstract
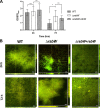
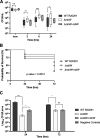
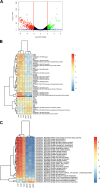
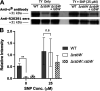
The anaerobic pathogen Clostridioides difficile, which is a primary cause of antibiotic-associated diarrhea, faces a variety of stresses in the environment and in the mammalian gut. To cope with these stresses, alternative sigma factor B (σB) is employed to modulate gene transcription, and σB is regulated by an anti-sigma factor, RsbW. To understand the role of RsbW in C. difficile physiology, a rsbW mutant (ΔrsbW), in which σB is assumed to be "always on," was generated. ΔrsbW did not show fitness defects in the absence of stress but tolerated acidic environments and detoxified reactive oxygen and nitrogen species better compared to the parental strain. ΔrsbW was defective in spore and biofilm formation, but it displayed increased adhesion to human gut epithelia and was less virulent in a Galleria mellonella infection model. A transcriptomic analysis to understand the unique phenotype of ΔrsbW showed changes in expression of genes associated with stress responses, virulence, sporulation, phage, and several σB-controlled regulators, including the pleiotropic regulator sinRR'. While these profiles were distinct to ΔrsbW, changes in some σB-controlled stress-associated genes were similar to those reported in the absence of σB. Further analysis of ΔrsbW showed unexpected lower intracellular levels of σB, suggesting an additional post-translational control mechanism for σB in the absence of stress. Our study provides insight into the regulatory role of RsbW and the complexity of regulatory networks mediating stress responses in C. difficile. IMPORTANCE Pathogens like Clostridioides difficile face a range of stresses in the environment and within the host. Alternative transcriptional factors like sigma factor B (σB) enable the bacterium to respond quickly to different stresses. Anti-sigma factors like RsbW control sigma factors and therefore the activation of genes via these pathways. Some of these transcriptional control systems provide C. difficile with the ability to tolerate and detoxify harmful compounds. Here, we investigate the role of RsbW in C. difficile physiology. We demonstrate distinctive phenotypes for a rsbW mutant in growth, persistence, and virulence and suggest alternate σB control mechanisms in C. difficile. Understanding C. difficile responses to external stress is key to designing better strategies to combat this highly resilient bacterial pathogen.
Keywords: Clostridioides difficile; anti-sigma factor RsbW; regulation; sigma factor B; stress responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Activation of the Extracytoplasmic Function σ Factor σV in Clostridioides difficile Requires Regulated Intramembrane Proteolysis of the Anti-σ Factor RsiV.mSphere. 2022 Apr 27;7(2):e0009222. doi: 10.1128/msphere.00092-22. Epub 2022 Mar 23. mSphere. 2022. PMID: 35317618 Free PMC article.
-
How the Anaerobic Enteropathogen Clostridioides difficile Tolerates Low O2 Tensions.mBio. 2020 Sep 8;11(5):e01559-20. doi: 10.1128/mBio.01559-20. mBio. 2020. PMID: 32900801 Free PMC article.
-
The σB signalling activation pathway in the enteropathogen Clostridioides difficile.Environ Microbiol. 2019 Aug;21(8):2852-2870. doi: 10.1111/1462-2920.14642. Epub 2019 May 15. Environ Microbiol. 2019. PMID: 31032549
-
Activation of the extracytoplasmic function σ factor σV by lysozyme in Clostridioides difficile.Curr Opin Microbiol. 2022 Feb;65:162-166. doi: 10.1016/j.mib.2021.11.008. Epub 2021 Dec 8. Curr Opin Microbiol. 2022. PMID: 34894542 Free PMC article. Review.
-
Regulatory transcription factors of Clostridioides difficile pathogenesis with a focus on toxin regulation.Crit Rev Microbiol. 2023 May;49(3):334-349. doi: 10.1080/1040841X.2022.2054307. Epub 2022 Apr 7. Crit Rev Microbiol. 2023. PMID: 35389761 Free PMC article. Review.
Cited by
-
Nonmammalian models to study Clostridioides difficile infection; a systematic review.Anaerobe. 2023 Feb;79:102694. doi: 10.1016/j.anaerobe.2023.102694. Epub 2023 Jan 7. Anaerobe. 2023. PMID: 36626950 Free PMC article.
-
Adaptation mechanisms of Clostridioides difficile to auranofin and its impact on human gut microbiota.NPJ Biofilms Microbiomes. 2024 Sep 17;10(1):86. doi: 10.1038/s41522-024-00551-3. NPJ Biofilms Microbiomes. 2024. PMID: 39284817 Free PMC article.
-
Distinct impacts of each anti-anti-sigma factor ortholog of the chlamydial Rsb partner switching mechanism on development in Chlamydia trachomatis.Microbiol Spectr. 2024 Oct 29;12(12):e0184624. doi: 10.1128/spectrum.01846-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 39470281 Free PMC article.
-
Clostridioides difficile infection study models and prospectives for probing the microbe-host interface.J Bacteriol. 2025 Mar 20;207(3):e0040724. doi: 10.1128/jb.00407-24. Epub 2025 Feb 6. J Bacteriol. 2025. PMID: 39912651 Free PMC article. Review.
References
-
- Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM, Wilson LE, Holzbauer SM, Phipps EC, Dumyati GK, Beldavs ZG, Kainer MA, Karlsson M, Gerding DN, McDonald LC, Emerging Infections Program Clostridioides difficile Infection Working Group . 2020. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 382:1320–1330. doi:10.1056/NEJMoa1910215. - DOI - PMC - PubMed
-
- Vardakas KZ, Polyzos KA, Patouni K, Rafailidis PI, Samonis G, Falagas ME. 2012. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. Int J Antimicrob Agents 40:1–8. doi:10.1016/j.ijantimicag.2012.01.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

