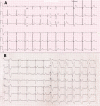
Transient left bundle branch block associated with favipiravir treatment for coronavirus infection
- PMID: 37099218
- PMCID: PMC10130810
- DOI: 10.1007/s10840-023-01548-2
Transient left bundle branch block associated with favipiravir treatment for coronavirus infection
Abstract
The coronavirus disease 2019 (COVID-19) may lead to a wide spectrum of clinical manifestations ranging from asymptomatic to symptomatic by having targets on various tissues such as lung parenchyma and myocardium (Shahrbaf et al., Cardiovasc Hematol Disord Drug Targets. 21(2):88-90, 2021). As an RNA-dependent RNApolymerase inhibitor, favipiravir has been proposed as a treatment in clinical studies done during the pandemic period (Furuta et al., Antiviral Res. 100(2):446-454, 2013). Although favipiravir is generally a safe medication, it may rarely cause cardiac adverse effects (Shahrbaf et al., Cardiovasc Hematol Disord Drug Targets. 21(2):88-90, 2021). To the best of our knowledge, favipiravir has not been reported to cause left bundle branch block (LBBB).
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Right, but not left, bundle branch block is associated with large anteroseptal scar.J Am Coll Cardiol. 2013 Sep 10;62(11):959-67. doi: 10.1016/j.jacc.2013.04.060. Epub 2013 May 22. J Am Coll Cardiol. 2013. PMID: 23707313 Free PMC article.
-
Evaluating Electrocardiography-Based Identification of Cardiac Resynchronization Therapy Responders Beyond Current Left Bundle Branch Block Definitions.JACC Clin Electrophysiol. 2020 Feb;6(2):193-203. doi: 10.1016/j.jacep.2019.10.009. Epub 2019 Nov 27. JACC Clin Electrophysiol. 2020. PMID: 32081223
-
["Left bundle branch block" pattern induced by premature right atrial stimulation. Electrogenetic and clinical considerations (author's transl)].G Ital Cardiol. 1981;11(8):1026-43. G Ital Cardiol. 1981. PMID: 6459963 Italian.
-
Bilateral Bundle Branch Block.Card Electrophysiol Clin. 2021 Dec;13(4):685-689. doi: 10.1016/j.ccep.2021.06.008. Epub 2021 Sep 23. Card Electrophysiol Clin. 2021. PMID: 34689895 Review.
-
Bilateral Bundle Branch Block.Cardiol Clin. 2023 Aug;41(3):393-397. doi: 10.1016/j.ccl.2023.03.011. Cardiol Clin. 2023. PMID: 37321689 Review.
Cited by
-
Favipiravir-induced bradycardia: A case report.Clin Case Rep. 2024 Jun 27;12(7):e9052. doi: 10.1002/ccr3.9052. eCollection 2024 Jul. Clin Case Rep. 2024. PMID: 38947534 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials