Intravenous Sodium Thiosulphate for Calciphylaxis of Chronic Kidney Disease: A Systematic Review and Meta-analysis
- PMID: 37099293
- PMCID: PMC10134003
- DOI: 10.1001/jamanetworkopen.2023.10068
Intravenous Sodium Thiosulphate for Calciphylaxis of Chronic Kidney Disease: A Systematic Review and Meta-analysis
Abstract
Importance: Calciphylaxis is a rare disease with high mortality mainly involving patients with chronic kidney disease (CKD). Sodium thiosulphate (STS) has been used as an off-label therapeutic in calciphylaxis, but there is a lack of clinical trials and studies that demonstrate its effect compared with those without STS treatment.
Objective: To perform a meta-analysis of the cohort studies that provided data comparing outcomes among patients with calciphylaxis treated with and without intravenous STS.
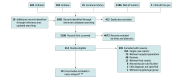
Data sources: PubMed, Embase, Cochrane Library, Web of Science, and ClinicalTrials.gov were searched using relevant terms and synonyms including sodium thiosulphate and calci* without language restriction.
Study selection: The initial search was for cohort studies published before August 31, 2021, that included adult patients diagnosed with CKD experiencing calciphylaxis and could provide a comparison between patients treated with and without intravenous STS. Studies were excluded if they reported outcomes only from nonintravenous administration of STS or if the outcomes for CKD patients were not provided.
Data extraction and synthesis: Random-effects models were performed. The Egger test was used to measure publication bias. Heterogeneity was assessed using the I2 test.
Main outcomes and measures: Skin lesion improvement and survival, synthesized as ratio data by a random-effects empirical Bayes model.
Results: Among the 5601 publications retrieved from the targeted databases, 19 retrospective cohort studies including 422 patients (mean age, 57 years; 37.3% male) met the eligibility criteria. No difference was observed in skin lesion improvement (12 studies with 110 patients; risk ratio, 1.23; 95% CI, 0.85-1.78) between the STS and the comparator groups. No difference was noted for the risk of death (15 studies with 158 patients; risk ratio, 0.88; 95% CI, 0.70-1.10) and overall survival using time-to-event data (3 studies with 269 participants; hazard ratio, 0.82; 95% CI, 0.57-1.18). In meta-regression, lesion improvement associated with STS negatively correlated with publication year, implying that recent studies are more likely to report a null association compared with past studies (coefficient = -0.14; P = .008).
Conclusions and relevance: Intravenous STS was not associated with skin lesion improvement or survival benefit in patients with CKD experiencing calciphylaxis. Future investigations are warranted to examine the efficacy and safety of therapies for patients with calciphylaxis.
Conflict of interest statement
Figures
References
-
- Kidney Disease: Improving Global Outcomes CKD-MBD Work Group . KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7(1):1-59. doi:10.1016/j.kisu.2017.04.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous