Efficacy and Safety of Low-Dose Triple and Quadruple Combination Pills vs Monotherapy, Usual Care, or Placebo for the Initial Management of Hypertension: A Systematic Review and Meta-analysis
- PMID: 37099314
- PMCID: PMC10134039
- DOI: 10.1001/jamacardio.2023.0720
Efficacy and Safety of Low-Dose Triple and Quadruple Combination Pills vs Monotherapy, Usual Care, or Placebo for the Initial Management of Hypertension: A Systematic Review and Meta-analysis
Abstract
Importance: Low-dose combination (LDC) antihypertensives consisting of 3 or 4 blood pressure (BP)-lowering drugs have emerged as a potentially important therapy for the initial management of hypertension.
Objective: To assess the efficacy and safety of LDC therapies for the management of hypertension.
Data sources: PubMed and Medline were searched from date of inception until September 2022.
Study selection: Randomized clinical trials comparing LDC consisting of 3 or 4 BP-lowering drugs compared to either monotherapy, usual care, or placebo.
Data extraction and synthesis: Data were extracted by 2 independent authors and synthesized using both random and fixed-effects models using risk ratios (RR) for binary outcomes and mean differences for continuous outcomes.
Main outcomes and measures: The primary outcome was mean reduction in systolic BP (SBP) between LDC and monotherapy, usual care, or placebo. Other outcomes of interest included the proportion of patients achieving BP less than 140/90 mm Hg, rates of adverse effects, and treatment withdrawal.
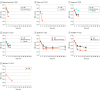
Results: Seven trials with a total of 1918 patients (mean [mean range] age, 59 [50-70] years; 739 [38%] female) were included. Four trials involved triple-component LDC and 3 involved quadruple-component LDC. At 4 to 12 weeks follow-up, LDC was associated with a greater mean reduction in SBP than initial monotherapy or usual care (mean reduction, 7.4 mm Hg; 95% CI, 4.3-10.5) and placebo (mean reduction, 18.0 mm Hg; 95% CI, 15.1-20.8). LDC was associated with a higher proportion of participants achieving BP less than 140/90 mm Hg at 4 to 12 weeks compared to both monotherapy or usual care (66% vs 46%; RR, 1.40; 95% CI, 1.27-1.52) and placebo (54% vs 18%; RR, 3.03; 95% CI, 1.93-4.77). There was no significant heterogeneity between trials enrolling patients with and without baseline BP-lowering therapy. Results from 2 trials indicated LDC remained superior to monotherapy or usual care at 6 to 12 months. LDC was associated with more dizziness (14% vs 11%; RR 1.28, 95% CI 1.00-1.63) but no other adverse effects nor treatment withdrawal.
Conclusions and relevance: The findings in the study showed that LDCs with 3 or 4 antihypertensives were an effective and well-tolerated BP-lowering treatment option for the initial or early management of hypertension.
Conflict of interest statement
Figures

References
-
- Chow CK, Teo KK, Rangarajan S, et al. ; PURE (Prospective Urban Rural Epidemiology) Study investigators . Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310(9):959-968. doi:10.1001/jama.2013.184182 - DOI - PubMed
-
- Whelton PK, Carey RM, Aronow WS, et al. . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

