Improving the hole picture: towards a consensus on the mechanism of nuclear transport
- PMID: 37099395
- PMCID: PMC10212546
- DOI: 10.1042/BST20220494
Improving the hole picture: towards a consensus on the mechanism of nuclear transport
Abstract
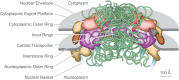
Nuclear pore complexes (NPCs) mediate the exchange of materials between the nucleoplasm and cytoplasm, playing a key role in the separation of nucleic acids and proteins into their required compartments. The static structure of the NPC is relatively well defined by recent cryo-EM and other studies. The functional roles of dynamic components in the pore of the NPC, phenylalanyl-glycyl (FG) repeat rich nucleoporins, is less clear because of our limited understanding of highly dynamic protein systems. These proteins form a 'restrained concentrate' which interacts with and concentrates nuclear transport factors (NTRs) to provide facilitated nucleocytoplasmic transport of cargoes. Very rapid on- and off-rates among FG repeats and NTRs supports extremely fast facilitated transport, close to the rate of macromolecular diffusion in cytoplasm, while complexes without specific interactions are entropically excluded, though details on several aspects of the transport mechanism and FG repeat behaviors remain to be resolved. However, as discussed here, new technical approaches combined with more advanced modeling methods will likely provide an improved dynamic description of NPC transport, potentially at the atomic level in the near future. Such advances are likely to be of major benefit in comprehending the roles the malfunctioning NPC plays in cancer, ageing, viral diseases, and neurodegeneration.
Keywords: cell nucleus; nuclear pores; nuclear protein transportport proteins; nucleic acids.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



Update of
-
Improving the Hole Picture: Towards a Consensus on the Mechanism of Nuclear Transport.ArXiv [Preprint]. 2023 Apr 6:arXiv:2304.03230v1. ArXiv. 2023. Update in: Biochem Soc Trans. 2023 Apr 26;51(2):871-886. doi: 10.1042/BST20220494. PMID: 37064528 Free PMC article. Updated. Preprint.
Similar articles
-
Improving the Hole Picture: Towards a Consensus on the Mechanism of Nuclear Transport.ArXiv [Preprint]. 2023 Apr 6:arXiv:2304.03230v1. ArXiv. 2023. Update in: Biochem Soc Trans. 2023 Apr 26;51(2):871-886. doi: 10.1042/BST20220494. PMID: 37064528 Free PMC article. Updated. Preprint.
-
C9orf72 polyPR interaction with the nuclear pore complex.Biophys J. 2024 Oct 15;123(20):3533-3539. doi: 10.1016/j.bpj.2024.08.024. Epub 2024 Aug 30. Biophys J. 2024. PMID: 39205388
-
Strategies for the Viral Exploitation of Nuclear Pore Transport Pathways.Viruses. 2025 Jan 23;17(2):151. doi: 10.3390/v17020151. Viruses. 2025. PMID: 40006906 Free PMC article. Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
Imaging-based quantitative assessment of biomolecular condensates in vitro and in cells.J Biol Chem. 2025 Feb;301(2):108130. doi: 10.1016/j.jbc.2024.108130. Epub 2024 Dec 24. J Biol Chem. 2025. PMID: 39725032 Free PMC article.
-
Regulating transport efficiency through the nuclear pore complex: The role of binding affinity with FG-Nups.Mol Biol Cell. 2024 Dec 1;35(12):ar149. doi: 10.1091/mbc.E24-05-0224. Epub 2024 Oct 30. Mol Biol Cell. 2024. PMID: 39475712 Free PMC article.
-
Elucidating the nanoscopic organization and dynamics of the nuclear pore complex.Nucleus. 2025 Dec;16(1):2510106. doi: 10.1080/19491034.2025.2510106. Epub 2025 Jun 4. Nucleus. 2025. PMID: 40464641 Free PMC article.
-
Coacervate-pore complexes for selective molecular transport and dynamic reconfiguration.Nat Commun. 2024 Nov 20;15(1):10069. doi: 10.1038/s41467-024-54510-9. Nat Commun. 2024. PMID: 39567561 Free PMC article.
-
The TEMPO integrator: accelerating molecular simulations by temporally multiscale force prediction.Bioinform Adv. 2025 Jun 20;5(1):vbaf142. doi: 10.1093/bioadv/vbaf142. eCollection 2025. Bioinform Adv. 2025. PMID: 40861395 Free PMC article.
References
-
- Chandra, B., Michmerhuizen, N.L., Shirnekhi, H.K., Tripathi, S., Pioso, B.J., Baggett, D.W.et al. (2022) Phase separation mediates NUP98 fusion oncoprotein leukemic transformation. Cancer Discov. 12, 1152–1169 10.1158/2159-8290.CD-21-0674 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

