Capillary driven microfluidic sequential flow device for point-of-need ELISA: COVID-19 serology testing
- PMID: 37099406
- PMCID: PMC10249653
- DOI: 10.1039/d3ay00225j
Capillary driven microfluidic sequential flow device for point-of-need ELISA: COVID-19 serology testing
Abstract
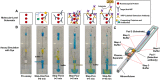
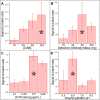
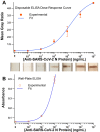
A capillary-driven microfluidic sequential flow device, designed for eventual at-home or doctor's office use, was developed to perform an enzyme-linked immunosorbent assay (ELISA) for serology assays. Serology assays that detect SARS-CoV-2 antibodies can be used to determine prior infection, immunity status, and/or individual vaccination status and are typically run using well-plate ELISAs in centralized laboratories, but in this format SARs-CoV-2 serology tests are too expensive and/or slow for most situations. Instead, a point-of-need device that can be used at home or in doctor's offices for COVID-19 serology testing would provide critical information for managing infections and determining immune status. Lateral flow assays are common and easy to use, but lack the sensitivity needed to reliably detect SARS-CoV-2 antibodies in clinical samples. This work describes a microfluidic sequential flow device that is as simple to use as a lateral flow assay, but as sensitive as a well-plate ELISA through sequential delivery of reagents to the detection area using only capillary flow. The device utilizes a network of microfluidic channels made of transparency film and double-sided adhesive combined with paper pumps to drive flow. The geometry of the channels and storage pads enables automated sequential washing and reagent addition steps with two simple end-user steps. An enzyme label and colorimetric substrate produce an amplified, visible signal for increased sensitivity, while the integrated washing steps decrease false positives and increase reproducibility. Naked-eye detection can be used for qualitative results or a smartphone camera for quantitative analysis. The device detected antibodies at 2.8 ng mL-1 from whole blood, while a well-plate ELISA using the same capture and detection antibodies could detect 1.2 ng mL-1. The performance of the capillary-driven immunoassay (CaDI) system developed here was confirmed by demonstrating SARS-CoV-2 antibody detection, and we believe that the device represents a fundamental step forward in equipment-free point-of-care technology.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Q. Diagnostics, COVID-19 test price information, https://www.questdiagnostics.com/business-solutions/health-plans/covid-1..., accessed March 13, 2022
-
- COVID-19 Target product profiles for priority diagnostics to support response to the COVID-19 pandemic v.0.1, World Health Organization, 2020
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

