Point-of-care ultrasonography for risk stratification of non-critical suspected COVID-19 patients on admission (POCUSCO): A prospective binational study
- PMID: 37099493
- PMCID: PMC10132646
- DOI: 10.1371/journal.pone.0284748
Point-of-care ultrasonography for risk stratification of non-critical suspected COVID-19 patients on admission (POCUSCO): A prospective binational study
Abstract
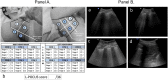
Background: Lung point-of-care ultrasonography (L-POCUS) is highly effective in detecting pulmonary peripheral patterns and may allow early identification of patients who are likely to develop an acute respiratory distress syndrome (ARDS). We hypothesized that L-POCUS performed within the first 48 hours of non-critical patients with suspected COVID-19 would identify those with a high-risk of worsening.
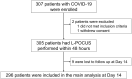
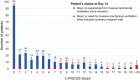
Methods: POCUSCO was a prospective, multicenter study. Non-critical adult patients who presented to the emergency department (ED) for suspected or confirmed COVID-19 were included and had L-POCUS performed within 48 hours following ED presentation. The lung damage severity was assessed using a previously developed score reflecting both the extension and the intensity of lung damage. The primary outcome was the rate of patients requiring intubation or who died within 14 days following inclusion.
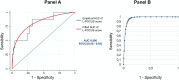
Results: Among 296 patients, 8 (2.7%) met the primary outcome. The area under the curve (AUC) of L-POCUS was 0.80 [95%CI:0.60-0.94]. The score values which achieved a sensibility >95% in defining low-risk patients and a specificity >95% in defining high-risk patients were <1 and ≥16, respectively. The rate of patients with an unfavorable outcome was 0/95 (0%[95%CI:0-3.9]) for low-risk patients (score = 0), 4/184 (2.17%[95%CI:0.8-5.5]) for intermediate-risk patients (score 1-15) and 4/17 (23.5%[95%CI:11.4-42.4]) for high-risk patients (score ≥16). In confirmed COVID-19 patients (n = 58), the AUC of L-POCUS was 0.97 [95%CI:0.92-1.00].
Conclusion: L-POCUS performed within the first 48 hours following ED presentation allows risk-stratification of patients with non-severe COVID-19.
Copyright: © 2023 Morin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Pr. Christophe Aubé declares personal scientific collaborations with Siemens Ultrasound, outside the submitted work. Pr. Francis Couturaud declares personal consulting fees and other from BMS, personal consulting fees and other from Bayer, personal consulting fees and other from MSD, outside the submitted work. Pr. Pierre-Marie Roy declares personal fees and other from Aspen, personal fees and other from Boehringer Ingelheim, personal fees and other from Bristol Myers Squibb, other from Bayer Health Care, outside the submitted work. Other authors declare no competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

