Mecom mutation related to radioulnar synostosis with amegakaryocytic thrombocytopenia reduces HSPCs in mice
- PMID: 37099686
- PMCID: PMC10509669
- DOI: 10.1182/bloodadvances.2022008462
Mecom mutation related to radioulnar synostosis with amegakaryocytic thrombocytopenia reduces HSPCs in mice
Abstract
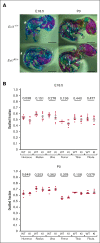
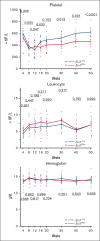
Radioulnar synostosis with amegakaryocytic thrombocytopenia (RUSAT) is an inherited bone marrow failure syndrome characterized by the congenital fusion of the forearm bones. RUSAT is largely caused by missense mutations that are clustered in a specific region of the MDS1 and EVI1 complex locus (MECOM). EVI1, a transcript variant encoded by MECOM, is a zinc finger transcription factor involved in hematopoietic stem cell maintenance that induce leukemic transformation when overexpressed. Mice with exonic deletions in Mecom show reduced hematopoietic stem and progenitor cells (HSPCs). However, the pathogenic roles of RUSAT-associated MECOM mutations in vivo have not yet been elucidated. To investigate the impact of the RUSAT-associated MECOM mutation on the phenotype, we generated knockin mice harboring a point mutation (translated into EVI1 p.H752R and MDS1-EVI1 p.H942R), which corresponds to an EVI1 p.H751R and MDS1-EVI1 p.H939R mutation identified in a patient with RUSAT. Homozygous mutant mice died at embryonic day 10.5 to 11.5. Heterozygous mutant mice (Evi1KI/+ mice) grew normally without radioulnar synostosis. Male Evi1KI/+ mice, aged between 5 and 15 weeks, exhibited lower body weight, and those aged ≥16 weeks showed low platelet counts. Flow cytometric analysis of bone marrow cells revealed a decrease in HSPCs in Evi1KI/+ mice between 8 and 12 weeks. Moreover, Evi1KI/+ mice showed delayed leukocyte and platelet recovery after 5-fluorouracil-induced myelosuppression. These findings suggest that Evi1KI/+ mice recapitulate the bone marrow dysfunction in RUSAT, similar to that caused by loss-of-function Mecom alleles.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures






References
-
- Bluteau O, Sebert M, Leblanc T, et al. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood. 2018;131(7):717–732. - PubMed
-
- Lord SV, Jimenez JE, Kroeger ZA, et al. A MECOM variant in an African American child with radioulnar synostosis and thrombocytopenia. Clin Dysmorphol. 2018;27(1):9–11. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

