Reducing uncertainties in quantitative adverse outcome pathways by analysis of thyroid hormone in the neonatal rat brain
- PMID: 37099719
- PMCID: PMC10732312
- DOI: 10.1093/toxsci/kfad040
Reducing uncertainties in quantitative adverse outcome pathways by analysis of thyroid hormone in the neonatal rat brain
Erratum in
-
Correction to: Reducing uncertainties in quantitative adverse outcome pathways by analysis of thyroid hormone in the neonatal rat brain.Toxicol Sci. 2023 Nov 28;196(2):263. doi: 10.1093/toxsci/kfad111. Toxicol Sci. 2023. PMID: 37931227 No abstract available.
Abstract
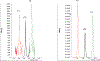
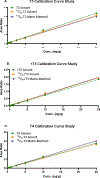
A number of xenobiotics interfere with thyroid hormone (TH) signaling. Although adequate supplies of TH are necessary for normal brain development, regulatory reliance on serum TH as proxies for brain TH insufficiency is fraught with significant uncertainties. A more direct causal linkage to neurodevelopmental toxicity induced by TH-system disrupting chemicals is to measure TH in the target organ of most concern, the brain. However, the phospholipid-rich matrix of brain tissue presents challenges for TH extraction and measurement. We report optimized analytical procedures to extract TH in brain tissue of rats with recoveries >80% and low detection limits for T3, rT3, and T4 (0.013, 0.033, and 0.028 ng/g, respectively). Recovery of TH is augmented by enhancing phospholipid separation from TH using an anion exchange column coupled with a stringent column wash. Quality control measures incorporating a matrix-matched calibration procedure revealed excellent recovery and consistency across a large number of samples. Application of optimized procedures revealed age-dependent increases in neonatal brain T4, T3, and rT3 on the day of birth (postnatal day, PN0), PN2, PN6, and PN14. No sex-dependent differences in brain TH were observed at these ages, and similar TH levels were evident in perfused versus non-perfused brains. Implementation of a robust and reliable method to quantify TH in the fetal and neonatal rat brain will aid in the characterization of the thyroid-dependent chemical interference on neurodevelopment. A brain- in addition to a serum-based metric will reduce uncertainties in assessment of hazard and risk on the developing brain posed by thyroid system-disrupting chemicals.
Keywords: adverse outcome pathway; brain; mass spectrometry; thyroid hormones.
Published by Oxford University Press on behalf of the Society of Toxicology 2023.
Figures


Similar articles
-
Neurodevelopment and Thyroid Hormone Synthesis Inhibition in the Rat: Quantitative Understanding Within the Adverse Outcome Pathway Framework.Toxicol Sci. 2017 Nov 1;160(1):57-73. doi: 10.1093/toxsci/kfx163. Toxicol Sci. 2017. PMID: 28973696 Free PMC article.
-
Thyroid Hormone Disruption in the Fetal and Neonatal Rat: Predictive Hormone Measures and Bioindicators of Hormone Action in the Developing Cortex.Toxicol Sci. 2018 Nov 1;166(1):163-179. doi: 10.1093/toxsci/kfy190. Toxicol Sci. 2018. PMID: 30085217 Free PMC article.
-
Fetal and neonatal iron deficiency exacerbates mild thyroid hormone insufficiency effects on male thyroid hormone levels and brain thyroid hormone-responsive gene expression.Endocrinology. 2014 Mar;155(3):1157-67. doi: 10.1210/en.2013-1571. Epub 2013 Dec 20. Endocrinology. 2014. PMID: 24424046 Free PMC article.
-
Disruption of the thyroid hormone system and patterns of altered thyroid hormones after gestational chemical exposures in rodents - a systematic review.Front Endocrinol (Lausanne). 2024 Jan 30;14:1323284. doi: 10.3389/fendo.2023.1323284. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38352246 Free PMC article.
-
Mechanisms of developmental neurotoxicity mediated by perturbed thyroid hormone homeostasis in the brain: an adverse outcome pathway network.Crit Rev Toxicol. 2025;55(3):304-320. doi: 10.1080/10408444.2025.2461076. Epub 2025 Mar 10. Crit Rev Toxicol. 2025. PMID: 40062460 Review.
Cited by
-
Two novel in vitro assays to screen chemicals for their capacity to inhibit thyroid hormone transmembrane transporter proteins OATP1C1 and OAT4.Arch Toxicol. 2024 Sep;98(9):3019-3034. doi: 10.1007/s00204-024-03787-2. Epub 2024 May 18. Arch Toxicol. 2024. PMID: 38761188 Free PMC article.
-
Optimal testing time for cerebral heterotopia formation in the rat comparative thyroid assay, a downstream indicator for perinatal thyroid hormone insufficiency.J Toxicol Pathol. 2024 Oct;37(4):173-187. doi: 10.1293/tox.2024-0004. Epub 2024 Jul 16. J Toxicol Pathol. 2024. PMID: 39359896 Free PMC article.
-
Structural Malformations in the Neonatal Rat Brain Accompany Developmental Exposure to Ammonium Perchlorate.Toxics. 2023 Dec 18;11(12):1027. doi: 10.3390/toxics11121027. Toxics. 2023. PMID: 38133428 Free PMC article.
-
A proposal of criteria to support the EU classification on endocrine disruption for the thyroid modality and their application to four data-rich case studies.Arch Toxicol. 2025 Jul;99(7):2727-2758. doi: 10.1007/s00204-025-04037-9. Epub 2025 May 10. Arch Toxicol. 2025. PMID: 40347277 Free PMC article.
-
The pollutant perfluorohexane sulfonate (PFHxS) reduces serum thyroxine but does not alter thyroid action in the postnatal rat brain.Environ Int. 2024 Aug;190:108838. doi: 10.1016/j.envint.2024.108838. Epub 2024 Jun 19. Environ Int. 2024. PMID: 38963985 Free PMC article.
References
-
- Axelstad M, Hansen PR, Boberg J, Bonnichsen M, Nellemann C, Lund SP, Hougaard KS, U H. 2008. Developmental neurotoxicity of propylthiouracil (ptu) in rats: Relationship between transient hypothyroxinemia during development and long-lasting behavioural and functional changes. Toxicol Appl Pharmacol. 232(1):1–13. - PubMed
-
- Baumgartner A, Pinna G, Hiedra L, Gaio U, Hessenius C, Campos-Barros A, Eravci M, Prengel H, Thoma R, Meinhold H. 1997. Effects of lithium and carbamazepine on thyroid hormone metabolism in rat brain. Neuropsychopharmacology. 16(1):25–41. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

