Adagrasib in Advanced Solid Tumors Harboring a KRASG12C Mutation
- PMID: 37099736
- PMCID: PMC10852394
- DOI: 10.1200/JCO.23.00434
Adagrasib in Advanced Solid Tumors Harboring a KRASG12C Mutation
Abstract
Purpose: Adagrasib, a KRASG12C inhibitor, has demonstrated clinical activity in patients with KRASG12C-mutated non-small-cell lung cancer (NSCLC) and colorectal cancer (CRC). KRASG12C mutations occur rarely in other solid tumor types. We report evaluation of the clinical activity and safety of adagrasib in patients with other solid tumors harboring a KRASG12C mutation.
Methods: In this phase II cohort of the KRYSTAL-1 study (ClinicalTrials.gov identifier: NCT03785249; phase Ib cohort), we evaluated adagrasib (600 mg orally twice daily) in patients with KRASG12C-mutated advanced solid tumors (excluding NSCLC and CRC). The primary end point was objective response rate. Secondary end points included duration of response, progression-free survival (PFS), overall survival, and safety.
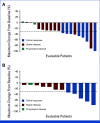
Results: As of October 1, 2022, 64 patients with KRASG12C-mutated solid tumors were enrolled and 63 patients treated (median follow-up, 16.8 months). The median number of prior lines of systemic therapy was 2. Among 57 patients with measurable disease at baseline, objective responses were observed in 20 (35.1%) patients (all partial responses), including 7/21 (33.3%) responses in pancreatic and 5/12 (41.7%) in biliary tract cancers. The median duration of response was 5.3 months (95% CI, 2.8 to 7.3) and median PFS was 7.4 months (95% CI, 5.3 to 8.6). Treatment-related adverse events (TRAEs) of any grade were observed in 96.8% of patients and grade 3-4 in 27.0%; there were no grade 5 TRAEs. TRAEs did not lead to treatment discontinuation in any patients.
Conclusion: Adagrasib demonstrates encouraging clinical activity and is well tolerated in this rare cohort of pretreated patients with KRASG12C-mutated solid tumors.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



Comment in
-
Cracking KRASG12C across all solid tumors: the new kid on the block for tissue-agnostic precision medicine.ESMO Open. 2023 Aug;8(4):101591. doi: 10.1016/j.esmoop.2023.101591. Epub 2023 Jun 30. ESMO Open. 2023. PMID: 37393631 Free PMC article. No abstract available.
References
-
- Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRAS (G12C) somatic mutations across race, sex, and cancer type. N Engl J Med. 2021;384:185–187. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

