The deubiquitinase USP11 ameliorates intervertebral disc degeneration by regulating oxidative stress-induced ferroptosis via deubiquitinating and stabilizing Sirt3
- PMID: 37099926
- PMCID: PMC10149406
- DOI: 10.1016/j.redox.2023.102707
The deubiquitinase USP11 ameliorates intervertebral disc degeneration by regulating oxidative stress-induced ferroptosis via deubiquitinating and stabilizing Sirt3
Abstract
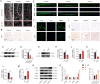
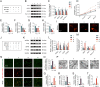
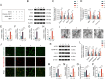
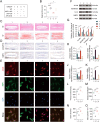
Increasing studies have reported that intervertebral disc degeneration (IVDD) is the main contributor and independent risk factor for low back pain (LBP), it would be, therefore, enlightening that investigating the exact pathogenesis of IVDD and developing target-specific molecular drugs in the future. Ferroptosis is a new form of programmed cell death characterized by glutathione (GSH) depletion, and inactivation of the regulatory core of the antioxidant system (glutathione system) GPX4. The close relationship of oxidative stress and ferroptosis has been studied in various of diseases, but the crosstalk between of oxidative stress and ferroptosis has not been explored in IVDD. At the beginning of the current study, we proved that Sirt3 decreases and ferroptosis occurs after IVDD. Next, we found that knockout of Sirt3 (Sirt3-/-) promoted IVDD and poor pain-related behavioral scores via increasing oxidative stress-induced ferroptosis. The (immunoprecipitation coupled with mass spectrometry) IP/MS and co-IP demonstrated that USP11 was identified to stabilize Sirt3 via directly binding to Sirt3 and deubiquitinating Sirt3. Overexpression of USP11 significantly ameliorate oxidative stress-induced ferroptosis, thus relieving IVDD by increasing Sirt3. Moreover, knockout of USP11 in vivo (USP11-/-) resulted in exacerbated IVDD and poor pain-related behavioral scores, which could be reversed by overexpression of Sirt3 in intervertebral disc. In conclusion, the current study emphasized the importance of the interaction of USP11 and Sirt3 in the pathological process of IVDD via regulating oxidative stress-induced ferroptosis, and USP11-mediated oxidative stress-induced ferroptosis is identified as a promising target for treating IVDD.
Keywords: De-ubiquitination; IVDD; Oxidative stress-induced ferroptosis; Sirt3; USP11.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors have declared that no competing interest exists.
Figures




References
-
- Morgan T., Wu J., Ovchinikova L., Lindner R., Blogg S., Moorin R. A national intervention to reduce imaging for low back pain by general practitioners: a retrospective economic program evaluation using Medicare Benefits Schedule data. BMC Health Serv. Res. 2019;19:983. doi: 10.1186/s12913-019-4773-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

