Maintenance of a short-lived protein required for long-term memory involves cycles of transcription and local translation
- PMID: 37100055
- PMCID: PMC10330212
- DOI: 10.1016/j.neuron.2023.04.005
Maintenance of a short-lived protein required for long-term memory involves cycles of transcription and local translation
Abstract
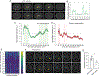
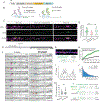
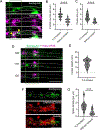
Activity-dependent expression of immediate early genes (IEGs) is critical for long-term synaptic remodeling and memory. It remains unknown how IEGs are maintained for memory despite rapid transcript and protein turnover. To address this conundrum, we monitored Arc, an IEG essential for memory consolidation. Using a knockin mouse where endogenous Arc alleles were fluorescently tagged, we performed real-time imaging of Arc mRNA dynamics in individual neurons in cultures and brain tissue. Unexpectedly, a single burst stimulation was sufficient to induce cycles of transcriptional reactivation in the same neuron. Subsequent transcription cycles required translation, whereby new Arc proteins engaged in autoregulatory positive feedback to reinduce transcription. The ensuing Arc mRNAs preferentially localized at sites marked by previous Arc protein, assembling a "hotspot" of translation, and consolidating "hubs" of dendritic Arc. These cycles of transcription-translation coupling sustain protein expression and provide a mechanism by which a short-lived event may support long-term memory.
Keywords: Arc; cyclical gene expression; immediate early genes; local translation; long-term memory; memory consolidation; protein hubs; single mRNA imaging; transcription; translation hotspots.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

