Conserved and cell type-specific transcriptional responses to IFN-γ in the ventral midbrain
- PMID: 37100211
- PMCID: PMC10460506
- DOI: 10.1016/j.bbi.2023.04.008
Conserved and cell type-specific transcriptional responses to IFN-γ in the ventral midbrain
Abstract
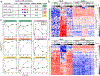
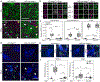
Dysregulated inflammation within the central nervous system (CNS) contributes to neuropathology in infectious, autoimmune, and neurodegenerative disease. With the exception of microglia, major histocompatibility complex (MHC) proteins are virtually undetectable in the mature, healthy central nervous system (CNS). Neurons have generally been considered incapable of antigen presentation, and although interferon gamma (IFN-γ) can elicit neuronal MHC class I (MHC-I) expression and antigen presentation in vitro, it has been unclear whether similar responses occur in vivo. Here we directly injected IFN-γ into the ventral midbrain of mature mice and analyzed gene expression profiles of specific CNS cell types. We found that IFN-γ upregulated MHC-I and associated mRNAs in ventral midbrain microglia, astrocytes, oligodendrocytes, and GABAergic, glutamatergic, and dopaminergic neurons. The core set of IFN-γ-induced genes and their response kinetics were similar in neurons and glia, but with a lower amplitude of expression in neurons. A diverse repertoire of genes was upregulated in glia, particularly microglia, which were the only cells to undergo cellular proliferation and express MHC classII (MHC-II) and associated genes. To determine if neurons respond directly via cell-autonomous IFN-γ receptor (IFNGR) signaling, we produced mutant mice with a deletion of the IFN-γ-binding domain of IFNGR1 in dopaminergic neurons, which resulted in a complete loss of dopaminergic neuronal responses to IFN-γ. Our results demonstrate that IFN-γ induces neuronal IFNGR signaling and upregulation of MHC-I and related genes in vivo, although the expression level is low compared to oligodendrocytes, astrocytes, and microglia.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G, 2000. Gene Ontology: tool for the unification of biology. Nat. Genet 25, 25–29. 10.1038/75556. - DOI - PMC - PubMed
-
- Barcia C, Ros CM, Annese V, Gómez A, Ros-Bernal F, Aguado-Yera D, Martínez-Pagán ME, de Pablos V, Fernandez-Villalba E, Herrero MT, 2011. IFN-γ signaling, with the synergistic contribution of TNF-α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis 2, e142. doi: 10.1038/cddis.2011.17. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

