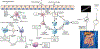Treatment of food allergy: Oral immunotherapy, biologics, and beyond
- PMID: 37100276
- PMCID: PMC10330596
- DOI: 10.1016/j.anai.2023.04.023
Treatment of food allergy: Oral immunotherapy, biologics, and beyond
Abstract
The prevalence of food allergy (FA) has been increasing globally and comes with a heavy burden not just economically, but also on quality of life. Although oral immunotherapy (OIT) is effective at inducing desensitization to food allergens, it has several limitations that weaken its success. Limitations include a long duration of build-up, especially when used for multiple allergens, and a high rate of reported adverse events. Furthermore, OIT may not be effective in all patients. Efforts are underway to identify additional treatment options, either as monotherapy or in combination, to treat FA or enhance the safety and efficacy of OIT. Biologics such as omalizumab and dupilumab, which already have US Food and Drug Administration approval for other atopic conditions have been the most studied, but additional biologics and novel strategies are emerging. In this review, we discuss therapeutic strategies including immunoglobulin E inhibitors, immunoglobulin E disruptors, interleukin-4 and interleukin-13 inhibitors, antialarmins, JAK1 and BTK inhibitors, and nanoparticles, and the data surrounding their application in FA and highlighting their potential.
Copyright © 2023 American College of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest:
Dr. Long reports consultant fees from COUR Pharmaceuticals. Dr. Chinthrajah reports grants from NIAID, CoFAR, Aimmune, DBV Technologies, Astellas, Regeneron, Stanford Maternal and Child Health Research Institute (MCHRI), and FARE. She is an Advisory Board Member at Alladapt Therapeutics, Novartis, Genentech, Sanofi, Allergenis, and Intrommune Therapeutics. Dr. Sindher reports grants from NIH, Regeneron, DBV Technologies, Aimmune, Novartis, CoFAR, and FARE. She is an Advisory member at Genentech and DBV Technologies. All other authors indicate no conflicts of interest.
Figures
References
-
- Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr 2013;167:1026–1031. - PubMed
-
- Shaker MS, Schwartz J, Ferguson M. An update on the impact of food allergy on anxiety and quality of life. Curr Opin Pediatr 2017;29:497–502. - PubMed
-
- Sindher SB, Long A, Chin AR, Hy A, Sampath V, Nadeau KC, et al. Food allergy, mechanisms, diagnosis and treatment: Innovation through a multi-targeted approach. Allergy 2022. - PubMed
-
- Investigators PGoC Vickery BP, Vereda A Casale TB, Beyer K, du Toit G, et al. AR101 Oral Immunotherapy for Peanut Allergy. N Engl J Med 2018;379:1991–2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous