A heme pocket aromatic quadrupole modulates gas binding to cytochrome c'-β: Implications for NO sensors
- PMID: 37100286
- PMCID: PMC10318465
- DOI: 10.1016/j.jbc.2023.104742
A heme pocket aromatic quadrupole modulates gas binding to cytochrome c'-β: Implications for NO sensors
Abstract
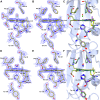
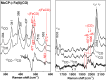
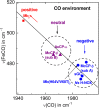
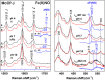
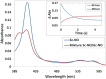
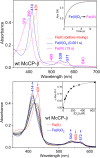
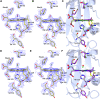
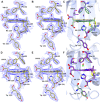
The structural basis by which gas-binding heme proteins control their interactions with NO, CO, and O2 is fundamental to enzymology, biotechnology, and human health. Cytochromes c' (cyts c') are a group of putative NO-binding heme proteins that fall into two families: the well-characterized four alpha helix bundle fold (cyts c'-α) and an unrelated family with a large beta-sheet fold (cyts c'-β) resembling that of cytochromes P460. A recent structure of cyt c'-β from Methylococcus capsulatus Bath revealed two heme pocket phenylalanine residues (Phe 32 and Phe 61) positioned near the distal gas-binding site. This feature, dubbed the "Phe cap," is highly conserved within the sequences of other cyts c'-β but is absent in their close homologs, the hydroxylamine-oxidizing cytochromes P460, although some do contain a single Phe residue. Here, we report an integrated structural, spectroscopic, and kinetic characterization of cyt c'-β from Methylococcus capsulatus Bath complexes with diatomic gases, focusing on the interaction of the Phe cap with NO and CO. Significantly, crystallographic and resonance Raman data show that orientation of the electron-rich aromatic ring face of Phe 32 toward distally bound NO or CO is associated with weakened backbonding and higher off rates. Moreover, we propose that an aromatic quadrupole also contributes to the unusually weak backbonding reported for some heme-based gas sensors, including the mammalian NO sensor, soluble guanylate cyclase. Collectively, this study sheds light on the influence of highly conserved distal Phe residues on heme-gas complexes of cytochrome c'-β, including the potential for aromatic quadrupoles to modulate NO and CO binding in other heme proteins.
Keywords: aromatic quadrupole; carbon monoxide; cytochrome; nitric oxide.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- Zahn J.A., Arciero D.M., Hooper A.B., Dispirito A.A. Cytochrome c′ of Methylococcus Capsulatus Bath. Eur. J. Biochem. 1996;240:684–691. - PubMed
-
- Poret-Peterson A.T., Graham J.E., Gulledge J., Klotz M.G. Transcription of nitrification genes by the methane-oxidizing bacterium, Methylococcus capsulatus strain Bath. ISME J. 2008;2:1213–1220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

