A model of Th2 differentiation based on polarizing cytokine repression
- PMID: 37100645
- PMCID: PMC10219849
- DOI: 10.1016/j.it.2023.04.004
A model of Th2 differentiation based on polarizing cytokine repression
Abstract
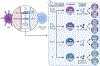
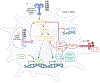
Conventional dendritic cells (cDCs) can integrate multiple stimuli from the environment and provide three separate outputs in terms of antigen presentation, costimulation, and cytokine production; this guides the activation, expansion, and differentiation of distinct functional T helper subsets. Accordingly, the current dogma posits that T helper cell specification requires these three signals in sequence. Data show that T helper 2 (Th2) cell differentiation requires antigen presentation and costimulation from cDCs but does not require polarizing cytokines. In this opinion article, we propose that the 'third signal' driving Th2 cell responses is, in fact, the absence of polarizing cytokines; indeed, the secretion of the latter is actively suppressed in cDCs, concomitant with acquired pro-Th2 functions.
Keywords: GATA3; IL-12; IL-2; IL-6; SOCS3; STAT5; T-bet; TGFβ; Th2 cells.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests No interests are declared.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

