Willingness of people with cystic fibrosis receiving elexacaftor/tezacaftor/ivacaftor (ETI) to participate in randomized modulator and inhaled antimicrobial clinical trials
- PMID: 37100705
- PMCID: PMC10523954
- DOI: 10.1016/j.jcf.2023.04.007
Willingness of people with cystic fibrosis receiving elexacaftor/tezacaftor/ivacaftor (ETI) to participate in randomized modulator and inhaled antimicrobial clinical trials
Abstract
Objective: To assess the feasibility of enrolling people with CF (pwCF) taking the CFTR modulator elexacaftor/tezacaftor/ivacaftor (ETI) in clinical trials of a new modulator.
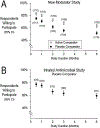
Methods: PwCF receiving ETI at CHEC-SC study (NCT03350828) enrollment were surveyed for interest in 2-week to 6-month placebo- (PC) and active-comparator (AC) modulator studies. Those taking inhaled antimicrobials (inhABX) were surveyed for interest in PC inhABX studies.
Results: Of 1791 respondents, 75% [95% CI 73, 77] would enroll in a 2-week PC modulator study versus 51% [49, 54] for a 6-month study; 82% [81, 84] and 63% [61, 65] would enroll in 2-week and 6 month AC studies; 77% [74, 80] of 551 taking inhABX would enroll in a 2-week PC inhABX study versus 59% [55, 63] for a 6-month study. Previous clinical trial experience increased willingness.
Conclusions: Study designs will affect feasibility of future clinical trials of new modulators and inhABX in people receiving ETI.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors have no competing or conflicting interests to report.
Figures
Comment in
-
Cystic fibrosis research: The only constant is change.J Cyst Fibros. 2023 Jul;22(4):596-597. doi: 10.1016/j.jcf.2023.07.008. Epub 2023 Sep 6. J Cyst Fibros. 2023. PMID: 37679269 No abstract available.
References
-
- Leonhardt K, Autry EB, Kuhn RJ, Wurth MA. CFTR modulator drug desensitization: Preserving the hope of long term improvement. Pediatr Pulmonol. 2021. Aug;56(8):2546–2552. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

