Mini-PCDH15 gene therapy rescues hearing in a mouse model of Usher syndrome type 1F
- PMID: 37100771
- PMCID: PMC10133396
- DOI: 10.1038/s41467-023-38038-y
Mini-PCDH15 gene therapy rescues hearing in a mouse model of Usher syndrome type 1F
Abstract
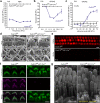
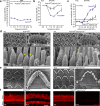
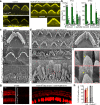
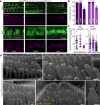
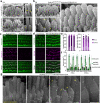
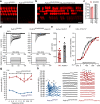
Usher syndrome type 1 F (USH1F), caused by mutations in the protocadherin-15 gene (PCDH15), is characterized by congenital deafness, lack of balance, and progressive blindness. In hair cells, the receptor cells of the inner ear, PCDH15 is a component of tip links, fine filaments which pull open mechanosensory transduction channels. A simple gene addition therapy for USH1F is challenging because the PCDH15 coding sequence is too large for adeno-associated virus (AAV) vectors. We use rational, structure-based design to engineer mini-PCDH15s in which 3-5 of the 11 extracellular cadherin repeats are deleted, but which still bind a partner protein. Some mini-PCDH15s can fit in an AAV. An AAV encoding one of these, injected into the inner ears of mouse models of USH1F, produces a mini-PCDH15 which properly forms tip links, prevents the degeneration of hair cell bundles, and rescues hearing. Mini-PCDH15s may be a useful therapy for the deafness of USH1F.
© 2023. The Author(s).
Conflict of interest statement
D.P.C. is a cofounder of Skylark Bio. M.V.I. is a consultant for Skylark Bio. President and Fellows of Harvard College, Ohio State Innovation Foundation, Massachusetts Eye and Ear Infirmary have filed patent application “AAV vectors encoding mini-PCDH15 and uses thereof”, inventors D.P.C., A.A.I., M.S., M.V.I. and C.W.P.; US (PCT/US2020/029968). The present disclosure provides isolated nucleic acids, vectors, and rAAV.9.PHP.B comprising a transgene encoding a mini-PCDH15, and methods of treating hearing loss using the same. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

