Isolating sensory artifacts in the suprathreshold TMS-EEG signal over DLPFC
- PMID: 37100795
- PMCID: PMC10130812
- DOI: 10.1038/s41598-023-29920-2
Isolating sensory artifacts in the suprathreshold TMS-EEG signal over DLPFC
Abstract
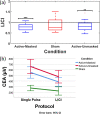
Combined transcranial magnetic stimulation and electroencephalography (TMS-EEG) is an effective way to evaluate neurophysiological processes at the level of the cortex. To further characterize the TMS-evoked potential (TEP) generated with TMS-EEG, beyond the motor cortex, we aimed to distinguish between cortical reactivity to TMS versus non-specific somatosensory and auditory co-activations using both single-pulse and paired-pulse protocols at suprathreshold stimulation intensities over the left dorsolateral prefrontal cortex (DLPFC). Fifteen right-handed healthy participants received six blocks of stimulation including single and paired TMS delivered as active-masked (i.e., TMS-EEG with auditory masking and foam spacing), active-unmasked (TMS-EEG without auditory masking and foam spacing) and sham (sham TMS coil). We evaluated cortical excitability following single-pulse TMS, and cortical inhibition following a paired-pulse paradigm (long-interval cortical inhibition (LICI)). Repeated measure ANOVAs revealed significant differences in mean cortical evoked activity (CEA) of active-masked, active-unmasked, and sham conditions for both the single-pulse (F(1.76, 24.63) = 21.88, p < 0.001, η2 = 0.61) and LICI (F(1.68, 23.49) = 10.09, p < 0.001, η2 = 0.42) protocols. Furthermore, global mean field amplitude (GMFA) differed significantly across the three conditions for both single-pulse (F(1.85, 25.89) = 24.68, p < 0.001, η2 = 0.64) and LICI (F(1.8, 25.16) = 14.29, p < 0.001, η2 = 0.5). Finally, only active LICI protocols but not sham stimulation ([active-masked (0.78 ± 0.16, P < 0.0001)], [active-unmasked (0.83 ± 0.25, P < 0.01)]) resulted in significant signal inhibition. While previous findings of a significant somatosensory and auditory contribution to the evoked EEG signal are replicated by our study, an artifact attenuated cortical reactivity can reliably be measured in the TMS-EEG signal with suprathreshold stimulation of DLPFC. Artifact attenuation can be accomplished using standard procedures, and even when masked, the level of cortical reactivity is still far above what is produced by sham stimulation. Our study illustrates that TMS-EEG of DLPFC remains a valid investigational tool.
© 2023. The Author(s).
Conflict of interest statement
ZJD has received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc and Magventure Inc. He is also on the scientific advisory board for Brainsway Inc. His work has been supported by the National Institutes of Mental Health (NIMH), the Canadian Institutes of Health Research (CIHR), Brain Canada and the Temerty Family, Grant and Kreutzcamp Family Foundations. DMB receives research support from CIHR, NIH, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he is the site principal investigator for one sponsor-initiated study for Brainsway Ltd. He also receives in-kind equipment support from Magventure for investigator-initiated studies. He received medication supplies for an investigator-initiated trial from Indivior. He participated in a Scientific Advisory Board for Janssen and Welcony Inc. C. Z. is a shareholder of sync2brain GmbH (Tübingen, Germany), a start-up commercializing real-time EEG analysis technology. TKR has received research support from Brain Canada, Brain and Behavior Research Foundation, BrightFocus Foundation, Canada Foundation for Innovation, Canada Research Chair, Canadian Institutes of Health Research, Centre for Aging and Brain Health Innovation, National Institutes of Health, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, and the Weston Brain Institute. TKR also received for an investigator-initiated study in-kind equipment support from Newronika, and in-kind research online accounts from Scientific Brain Training Pro, and participated in 2021 in an advisory board for Biogen Canada Inc. DV holds the Labatt Family Professorship in Depression Biology, a University Named Professorship at the University of Toronto. She receives research support from CIHR, the Centre for Addiction and Mental Health (CAMH) and the Department of Psychiatry at the University of Toronto. DV declares no biomedical interests or conflicts. MP, RZ, CH, ATH, IH, and RC have nothing to disclose.
Figures







References
-
- Ilmoniemi, R. J. et al. Dealing with artifacts in TMS-evoked EEG. in 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 230–233 (IEEE, 2015). 10.1109/EMBC.2015.7318342. - PubMed

