High glucose-induced STING activation inhibits diabetic wound healing through promoting M1 polarization of macrophages
- PMID: 37100799
- PMCID: PMC10133226
- DOI: 10.1038/s41420-023-01425-x
High glucose-induced STING activation inhibits diabetic wound healing through promoting M1 polarization of macrophages
Abstract
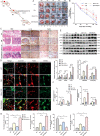
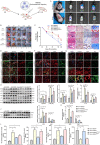
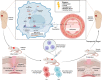
Diabetic wound (DW) is characterized by elevated pro-inflammatory cytokines and cellular dysfunction consistent with elevated reactive oxygen species (ROS) levels. Recent advances in immunology have dissected molecular pathways involved in the innate immune system where cytoplasmic DNA can trigger STING-dependent inflammatory responses and play an important role in metabolic-related diseases. We investigated whether STING regulates inflammation and cellular dysfunction in DW healing. We found that STING and M1 macrophages were increased in wound tissues from DW in patients and mice and delayed the wound closure. We also noticed that the massively released ROS in the High glucose (HG) environment activated STING signaling by inducing the escape of mtDNA to the cytoplasm, inducing macrophage polarization into a pro-inflammatory phenotype, releasing pro-inflammatory cytokines, and exacerbating endothelial cell dysfunction. In Conclusion, mtDNA-cGAS-STING pathway activation under diabetic metabolic stress is an important mechanism of DW refractory healing. While using STING gene-edited macrophages for wound treatment by cell therapy can induce the polarization of wound macrophages from pro-inflammatory M1 to anti-inflammatory M2, promote angiogenesis, and collagen deposition to accelerate DW healing. STING may be a promising therapeutic target for DW.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Loureirin B Accelerates Diabetic Wound Healing by Promoting TGFβ/Smad-Dependent Macrophage M2 Polarization: A Concerted Analytical Approach Through Single-Cell RNA Sequencing and Experimental Verification.Phytother Res. 2024 Nov 12. doi: 10.1002/ptr.8373. Online ahead of print. Phytother Res. 2024. PMID: 39532388
-
High glucose-induced endothelial STING activation inhibits diabetic wound healing through impairment of angiogenesis.Biochem Biophys Res Commun. 2023 Aug 6;668:82-89. doi: 10.1016/j.bbrc.2023.05.081. Epub 2023 May 22. Biochem Biophys Res Commun. 2023. PMID: 37245293
-
Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing.J Cell Physiol. 2019 Apr;234(4):4217-4231. doi: 10.1002/jcp.27185. Epub 2018 Aug 21. J Cell Physiol. 2019. PMID: 30132863
-
Innate Immunity in Diabetic Wound Healing: Focus on the Mastermind Hidden in Chronic Inflammatory.Front Pharmacol. 2021 Apr 21;12:653940. doi: 10.3389/fphar.2021.653940. eCollection 2021. Front Pharmacol. 2021. PMID: 33967796 Free PMC article. Review.
-
Regulation of Macrophage Polarization and Wound Healing.Adv Wound Care (New Rochelle). 2012 Feb;1(1):10-16. doi: 10.1089/wound.2011.0307. Adv Wound Care (New Rochelle). 2012. PMID: 24527272 Free PMC article. Review.
Cited by
-
Resveratrol Promotes Wound Healing by Enhancing Angiogenesis via Inhibition of Ferroptosis.Food Sci Nutr. 2025 May 5;13(5):e70254. doi: 10.1002/fsn3.70254. eCollection 2025 May. Food Sci Nutr. 2025. PMID: 40330211 Free PMC article.
-
Multimodal Synergistic Strategies for Diabetic Wound Healing Using Glucose Oxidase Nanocomposites: Therapeutic Mechanisms and Nanomaterial Design.Int J Nanomedicine. 2025 May 2;20:5727-5762. doi: 10.2147/IJN.S515057. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40337147 Free PMC article.
-
SZC-6 Promotes Diabetic Wound Healing in Mice by Modulating the M1/M2 Macrophage Ratio and Inhibiting the MyD88/NF-χB Pathway.Pharmaceuticals (Basel). 2025 Jul 31;18(8):1143. doi: 10.3390/ph18081143. Pharmaceuticals (Basel). 2025. PMID: 40872534 Free PMC article.
-
The cGAS-STING pathway: a therapeutic target in diabetes and its complications.Burns Trauma. 2024 Feb 2;12:tkad050. doi: 10.1093/burnst/tkad050. eCollection 2024. Burns Trauma. 2024. PMID: 38312740 Free PMC article. Review.
-
Advances in the prerequisite and consequence of STING downstream signalosomes.Med Rev (2021). 2024 May 8;4(5):435-451. doi: 10.1515/mr-2024-0016. eCollection 2024 Oct. Med Rev (2021). 2024. PMID: 39444795 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

