Early intervention with azelastine nasal spray may reduce viral load in SARS-CoV-2 infected patients
- PMID: 37100830
- PMCID: PMC10132439
- DOI: 10.1038/s41598-023-32546-z
Early intervention with azelastine nasal spray may reduce viral load in SARS-CoV-2 infected patients
Abstract
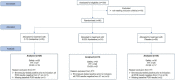
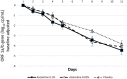
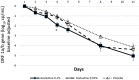
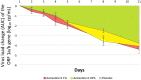
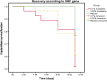
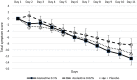
With the changing epidemiology of COVID-19 and its impact on our daily lives, there is still an unmet need of COVID-19 therapies treating early infections to prevent progression. The current study was a randomized, parallel, double-blind, placebo-controlled trial. Ninety SARS-CoV-2 positive patients were randomized into 3 groups receiving placebo, 0.02% or 0.1% azelastine nasal spray for 11 days, during which viral loads were assessed by quantitative PCR. Investigators assessed patients' status throughout the trial including safety follow-ups (days 16 and 60). Symptoms were documented in patient diaries. Initial viral loads were log10 6.85 ± 1.31 (mean ± SD) copies/mL (ORF 1a/b gene). After treatment, virus load was reduced in all groups (p < 0.0001) but was greater in the 0.1% group compared to placebo (p = 0.007). In a subset of patients (initial Ct < 25) viral load was strongly reduced on day 4 in the 0.1% group compared to placebo (p = 0.005). Negative PCR results appeared earlier and more frequently in the azelastine treated groups: being 18.52% and 21.43% in the 0.1% and 0.02% groups, respectively, compared to 0% for placebo on day 8. Comparable numbers of adverse events occurred in all treatment groups with no safety concerns. The shown effects of azelastine nasal spray may thus be suggestive of azelastine's potential as an antiviral treatment.Trial registration: The study was registered in the German Clinical Trial Register (DRKS-ID: DRKS00024520; Date of Registration in DRKS: 12/02/2021). EudraCT number: 2020-005544-34.
© 2023. The Author(s).
Conflict of interest statement
JPK and CL have received grants from the sponsor URSAPHARM Arzneimittel GmbH for performing this trial. EN, VS and GN are shareholders in CEBINA GmbH, RK and EN are inventors on related patent applications. PM, MF, DG, CS and BS are employed at URSAPHARM Arzneimittel GmbH. FH is the CEO of URSAPHARM Arzneimittel GmbH. BR, SMS, HS, CA, NW, SA, and RM are employees of ClinCompetence Cologne, the CRO which organized this trial. HG, MS, and FK declare no conflict of interest. MG, PA, HM and HAS declare no conflict of interest. AB is employed at Ursatec GmbH, supplier of primary packing materials to Ursapharm.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

