Evaluation of DNA methylation biomarkers ASCL1 and LHX8 on HPV-positive self-collected samples from primary HPV-based screening
- PMID: 37100874
- PMCID: PMC10132796
- DOI: 10.1038/s41416-023-02277-z
Evaluation of DNA methylation biomarkers ASCL1 and LHX8 on HPV-positive self-collected samples from primary HPV-based screening
Abstract
Background: Host-cell DNA methylation analysis can be used to triage women with high-risk human papillomavirus (HPV)-positive self-collected cervicovaginal samples, but current data are restricted to under-/never-screened women and referral populations. This study evaluated triage performance in women who were offered primary HPV self-sampling for cervical cancer screening.
Methods: Self-collected samples from 593 HPV-positive women who participated in a primary HPV self-sampling trial (IMPROVE study; NTR5078), were tested for the DNA methylation markers ASCL1 and LHX8 using quantitative multiplex methylation-specific PCR (qMSP). The diagnostic performance for CIN3 and cervical cancer (CIN3 + ) was evaluated and compared with that of paired HPV-positive clinician-collected cervical samples.
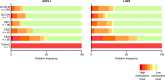
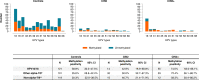
Results: Significantly higher methylation levels were found in HPV-positive self-collected samples of women with CIN3 + than control women with no evidence of disease (P values <0.0001). The marker panel ASCL1/LHX8 yielded a sensitivity for CIN3 + detection of 73.3% (63/86; 95% CI 63.9-82.6%), with a corresponding specificity of 61.1% (310/507; 95% CI 56.9-65.4%). The relative sensitivity for detecting CIN3+ was 0.95 (95% CI 0.82-1.10) for self-collection versus clinician-collection, and the relative specificity was 0.82 (95% CI 0.75-0.90).
Conclusions: The ASCL1/LHX8 methylation marker panel constitutes a feasible direct triage method for the detection of CIN3 + in HPV-positive women participating in routine screening by self-sampling.
© 2023. The Author(s).
Conflict of interest statement
DAMH, RDMS and CJLMM are minority shareholders of Self-screen B.V., a spin-off company of Amsterdam UMC, location VUmc; Self-screen B.V. develops, manufactures and licences high-risk HPV and methylation marker assays for cervical cancer screening and holds patents on these tests; JB had financial support from the European Commission (RISCC, grant number 847845); CJLMM is part-time CEO of Self-screen B.V., and has a very small number of shares of MDXHealth and previously QIAGEN, has received speakers fees from GSK, QIAGEN and SPMSD/Merck, and served occasionally on the scientific advisory boards of these companies; LV, MCGB, NP, RMFE, WJGM, RLMB, ACM, WGQ and FJvK declare no competing interests.
Figures


References
-
- Wong EL-Y, Wong CN-S, Cheung AW-L, Wong AY-K, Tam ZP-Y. Feasibility of human papillomavirus self-sampling to combat COVID-19-related disruptions to cervical cancer screening: a cross-sectional survey. Lancet Oncol. 2022;23:S16. doi: 10.1016/S1470-2045(22)00415-6. - DOI
-
- Polman NJ, Ebisch RMF, Heideman DAM, Melchers WJG, Bekkers RLM, Molijn AC, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229–38.. doi: 10.1016/S1470-2045(18)30763-0. - DOI - PubMed

