Click chemistry: a transformative technology in nuclear medicine
- PMID: 37100960
- PMCID: PMC10293801
- DOI: 10.1038/s41596-023-00825-8
Click chemistry: a transformative technology in nuclear medicine
Abstract
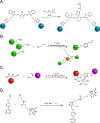
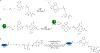
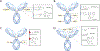
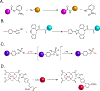
The 2022 Nobel Prize in Chemistry was awarded to Professors K. Barry Sharpless, Morten Meldal and Carolyn Bertozzi for their pioneering roles in the advent of click chemistry. Sharpless and Meldal worked to develop the canonical click reaction-the copper-catalyzed azide-alkyne cycloaddition-while Bertozzi opened new frontiers with the creation of the bioorthogonal strain-promoted azide-alkyne cycloaddition. These two reactions have revolutionized chemical and biological science by facilitating selective, high yielding, rapid and clean ligations and by providing unprecedented ways to manipulate living systems. Click chemistry has affected every aspect of chemistry and chemical biology, but few disciplines have been impacted as much as radiopharmaceutical chemistry. The importance of speed and selectivity in radiochemistry make it an almost tailor-made application of click chemistry. In this Perspective, we discuss the ways in which the copper-catalyzed azide-alkyne cycloaddition, the strain-promoted azide-alkyne cycloaddition and a handful of 'next-generation' click reactions have transformed radiopharmaceutical chemistry, both as tools for more efficient radiosyntheses and as linchpins of technologies that have the potential to improve nuclear medicine.
© 2023. Springer Nature Limited.
Figures
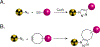
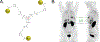

References
-
- The Nobel Prize in Chemistry 2022., <www.nobelprize.org/prizes/chemistry/2022/summary> (2022).
-
- Huisgen R Centenary Lecture - 1,3-Dipolar Cycloadditions. Proc. Chem. Soc, 357–396, doi: 10.1039/ps9610000357 (1961). - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

