Impact of pharmacogenomic DPYD variant guided dosing on toxicity in patients receiving fluoropyrimidines for gastrointestinal cancers in a high-volume tertiary centre
- PMID: 37101114
- PMCID: PMC10131438
- DOI: 10.1186/s12885-023-10857-8
Impact of pharmacogenomic DPYD variant guided dosing on toxicity in patients receiving fluoropyrimidines for gastrointestinal cancers in a high-volume tertiary centre
Abstract
Background: Dihydropyrimidine dehydrogenase (DPD) is a key enzyme in the metabolism of fluoropyrimidines. Variations in the encoding DPYD gene are associated with severe fluoropyrimidine toxicity and up-front dose reductions are recommended. We conducted a retrospective study to evaluate the impact of implementing DPYD variant testing for patients with gastrointestinal cancers in routine clinical practice in a high volume cancer centre in London, United Kingdom.
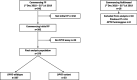
Methods: Patients receiving fluoropyrimidine chemotherapy for gastrointestinal cancer prior to, and following the implementation of DPYD testing were identified retrospectively. After November 2018, patients were tested for DPYD variants c.1905+1G>A (DPYD*2A), c.2846A>T (DPYD rs67376798), c.1679T>G (DPYD*13), c.1236G>A (DPYD rs56038477), c.1601G>A (DPYD*4) prior to commencing fluoropyrimidines alone or in combination with other cytotoxics and/or radiotherapy. Patients with a DPYD heterozygous variant received an initial dose reduction of 25-50%. Toxicity by CTCAE v4.03 criteria was compared between DPYD heterozygous variant and wild type carriers.
Results: Between 1st December 2018 and 31st July 2019, 370 patients who were fluoropyrimidine naïve underwent a DPYD genotyping test prior to receiving a capecitabine (n = 236, 63.8%) or 5FU (n = 134, 36.2%) containing chemotherapy regimen. 33 patients (8.8%) were heterozygous DPYD variant carriers and 337 (91.2%) were wild type. The most prevalent variants were c.1601G > A (n = 16) and c.1236G > A (n = 9). Mean relative dose intensity for the first dose was 54.2% (range 37.5-75%) for DPYD heterozygous carriers and 93.2% (42.9-100%) for DPYD wild type carriers. Overall grade 3 or worse toxicity was similar in DPYD variant carriers (4/33, 12.1%) as compared to wild-type carriers (89/337, 25.7%; P = 0.0924).
Conclusions: Our study demonstrates successful routine DPYD mutation testing prior to the initiation of fluoropyrimidine chemotherapy with high uptake. In patients with DPYD heterozygous variants with pre-emptive dose reductions, high incidence of severe toxicity was not observed. Our data supports routine DPYD genotype testing prior to commencement of fluoropyrimidine chemotherapy.
Keywords: 5-fluorouracil; Capecitabine; DPYD; Dihydropyrimidine dehydrogenase; Pharmacogenomics.
© 2023. The Author(s).
Conflict of interest statement
DL is the recipient of the Australasian Gastro-Intestinal Trials Group/Merck Clinical Research Fellowship. SR has served in a consulting role or on the advisory boards for Bayer, Boehringer Ingelheim, Hookipa Biotech, Incyte and Merck Serono.IC has served in a consulting role or on the advisory boards for Eli Lilly, Bristol Meyers Squibb, MSD, Bayer, Roche, Merck Serono, Five Prime Therapeutics, Astra Zeneca, OncXerna, Pierre Fabre, Boehringer Ingelheim, Incyte, Astellas, GSK, Sotio, Eisai and Daiichi Sankyo; received research funding from Eli Lilly, Janssen-Cilag; and honoraria from Eli Lilly, Eisai and Servier.DC has served in a consulting role or on the advisory boards for OVIBIO; received institutional research funding from 4SC, Bayer, Celgene, Clovis Oncology, Leap Oncology, Eli Lilly, MedImmune, Roche; and has ownership interests in OVIBIO.NS has served in a consulting role or on the advisory boards for AstraZeneca, MSD, Pfizer, Servier; received institutional research funding from AstraZeneca, BMS, Pfizer/ EMD Serono; honoraria from Amgen, GlaxoSmithKline, Lilly, Merck Serono, MSD Oncology, Pierre Fabre, Servier; and travel accommodation expenses from AstraZeneca, BMS, Lilly, Merck, MSD Oncology and Roche. C Fong, FA, LC, HK, RGE, MBR, SL, AMD, MAH, MH, C Fribbens and DW have no disclosures.
Figures
References
-
- Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS SSO and TOS. Ann Oncol. 2018;29(1):44–70. doi: 10.1093/annonc/mdx738. - DOI - PubMed
-
- NCCN: Colon Cancer v2.2021. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 3 Dec 2021.
-
- Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1462–1475. doi: 10.1016/j.annonc.2020.07.011. - DOI - PubMed
-
- Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, Dent R, Fenlon D, Gligorov J, Hurvitz SA, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer<sup>☆</sup>. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. - DOI - PubMed
-
- Froehlich TK, Amstutz U, Aebi S, Joerger M, Largiadèr CR. Clinical importance of risk variants in the dihydropyrimidine dehydrogenase gene for the prediction of early-onset fluoropyrimidine toxicity. Int J Cancer. 2015;136(3):730–739. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources