Superatomic Au25(SC2H5)18 Nanocluster under Pressure
- PMID: 37101514
- PMCID: PMC10114650
- DOI: 10.1021/acsnanoscienceau.1c00024
Superatomic Au25(SC2H5)18 Nanocluster under Pressure
Abstract
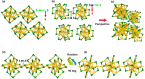
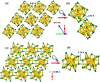
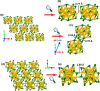
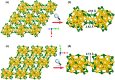
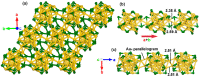
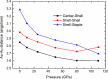
The past decade has witnessed significant advances in the synthesis and structure determination of atomically precise metal nanoclusters. However, little is known about the condensed matter properties of these nanosized metal nanoclusters packed in a crystal lattice under high pressure. Here using density functional theory calculations, we simulate the crystal of a representative superatomic gold cluster, Au25(SR)18 0 (R = C2H5), under various pressures. At ambient conditions, Au25(SC2H5)18 0 clusters are packed in a crystal via dispersion interactions; being a 7e superatom, each cluster carries a magnetic moment of 1 μB or one unpaired electron. Upon increasing compression (from 10 to 110 GPa), we observe the formation of intercluster Au-Au, Au-S, and S-S covalent bonds between staple motifs, thereby linking the clusters into a network. The pressure-induced structural change is accompanied by the vanishment of the magnetic moment and the semiconductor-to-metal transition. Our work shows that subjecting crystals of atomically precise metal nanoclusters to high pressures could lead to new crystalline states and physical properties.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Insights into Interfaces, Stability, Electronic Properties, and Catalytic Activities of Atomically Precise Metal Nanoclusters from First Principles.Acc Chem Res. 2018 Nov 20;51(11):2793-2802. doi: 10.1021/acs.accounts.8b00380. Epub 2018 Nov 6. Acc Chem Res. 2018. PMID: 30398051
-
Electrochemistry of Atomically Precise Metal Nanoclusters.Acc Chem Res. 2019 Jan 15;52(1):12-22. doi: 10.1021/acs.accounts.8b00379. Epub 2018 Nov 30. Acc Chem Res. 2019. PMID: 30500153
-
From superatomic Au25(SR)18(-) to superatomic M@Au24(SR)18(q) core-shell clusters.Inorg Chem. 2009 Apr 6;48(7):2720-2. doi: 10.1021/ic8024588. Inorg Chem. 2009. PMID: 19236016
-
Au25(SR)18: the captain of the great nanocluster ship.Nanoscale. 2018 Jun 14;10(23):10758-10834. doi: 10.1039/c8nr02973c. Nanoscale. 2018. PMID: 29873658 Review.
-
Metal Nanoclusters Stabilized by Selenol Ligands.Small. 2019 Oct;15(43):e1902703. doi: 10.1002/smll.201902703. Epub 2019 Sep 4. Small. 2019. PMID: 31482648 Review.
References
-
- Drozdov A. P.; Kong P. P.; Minkov V. S.; Besedin S. P.; Kuzovnikov M. A.; Mozaffari S.; Balicas L.; Balakirev F. F.; Graf D. E.; Prakapenka V. B.; Greenberg E.; Knyazev D. A.; Tkacz M.; Eremets M. I. Superconductivity at 250 K in lanthanum hydride under high pressures. Nature 2019, 569, 528–531. 10.1038/s41586-019-1201-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
