Fremanezumab for Chronic Migraine Prevention in Japanese Patients: Subgroup Analysis from Two International Trials
- PMID: 37101521
- PMCID: PMC10124568
- DOI: 10.2147/JPR.S393854
Fremanezumab for Chronic Migraine Prevention in Japanese Patients: Subgroup Analysis from Two International Trials
Abstract
Purpose: Fremanezumab monoclonal antibody therapy has demonstrated efficacy for chronic migraine (CM) with rapid onset and good tolerability. This subgroup analysis of two clinical trials (Japanese and Korean CM Phase 2b/3 [NCT03303079] and HALO CM Phase 3 [NCT02621931]) aimed to evaluate the efficacy and safety of fremanezumab in Japanese patients.
Patients and methods: Both trials randomly assigned eligible patients at baseline (1:1:1 ratio) to subcutaneous monthly fremanezumab, quarterly fremanezumab, or placebo at 4-week intervals. The primary endpoint was the mean change from baseline in the monthly (28-day) average number of headache days of at least moderate severity during the 12-week period after the first dose of study medication (analyzed by ANCOVA over 12 weeks and MMRM over initial 4 weeks). Secondary endpoints examined other aspects of efficacy, including medication use and disability.
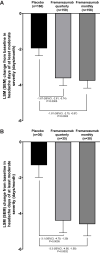
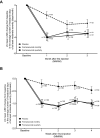
Results: A total of 479 and 109 patients were Japanese in the Japanese and Korean CM Phase 2b/3 and HALO CM trials, respectively. Baseline and treatment characteristics were generally similar between treatment groups for both trials. Results of subgroup analyses for the primary endpoint according to ANCOVA demonstrated the superiority of fremanezumab over placebo in Japanese patients (quarterly fremanezumab, p=0.0005; monthly fremanezumab, p=0.0002 in both trials). Results using the MMRM analysis confirmed the rapid onset of action in this population. Results of the secondary endpoints further supported the efficacy of fremanezumab in Japanese patients. Fremanezumab was well tolerated with nasopharyngitis and injection-site reactions representing the most common adverse events in all treatment groups.
Conclusion: Despite the limitations of subgroup analyses, these consistent results confirm the efficacy and tolerability of fremanezumab in Japanese patients with CM.
Keywords: Japanese; calcitonin gene-related peptide; chronic migraine; fremanezumab.
© 2023 Saigoh et al.
Conflict of interest statement
KS has received grants from Sumitomo Pharma Co., Ltd.; consulting fees from Takeda Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd.; payment for lectures from AMGEN Inc., Daiichi Sankyo Company, Eisai Co., Ltd., Eli Lilly Japan, Otsuka Pharmaceutical Co., Ltd., Sanofi Japan, Sumitomo Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd.; committee member for Japanese Society of Headache, Japanese Society of Human Genetics. TT has received honoraria for lectures from Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Amgen K.K., Eli Lilly and Company, Daiichi Sankyo; research funds under contract from Eisai Co., Ltd., Eli Lilly and Company, Amgen K.K., Allergan Japan K.K., Shionogi & Co., Ltd., Lundbeck Japan K.K. MN, YS, MI, YI, and NK are full-time employees of Otsuka Pharmaceutical Co., Ltd. XN and SB are full-time employees of Teva Branded Pharmaceutical Products. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Fremanezumab for Episodic Migraine Prevention in Japanese Patients: Subgroup Analysis from Two International Trials.J Pain Res. 2023 May 18;16:1673-1682. doi: 10.2147/JPR.S393896. eCollection 2023. J Pain Res. 2023. PMID: 37223438 Free PMC article.
-
Efficacy and safety of fremanezumab for chronic migraine prevention: Multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in Japanese and Korean patients.Headache. 2021 Jul;61(7):1092-1101. doi: 10.1111/head.14169. Epub 2021 Jul 29. Headache. 2021. PMID: 34324700 Free PMC article. Clinical Trial.
-
Reduction in the severity and duration of headache following fremanezumab treatment in patients with episodic and chronic migraine.Headache. 2021 Jun;61(6):916-926. doi: 10.1111/head.14127. Epub 2021 Jun 11. Headache. 2021. PMID: 34115380 Free PMC article. Clinical Trial.
-
Fremanezumab: a disease-specific option for the preventive treatment of migraine, including difficult-to-treat migraine.Emerg Top Life Sci. 2020 Sep 8;4(2):179-190. doi: 10.1042/ETLS20200018. Emerg Top Life Sci. 2020. PMID: 32832978 Free PMC article. Review.
-
Monthly versus quarterly fremanezumab for the prevention of migraine: a systemic review and meta-analysis from randomized controlled trials.Naunyn Schmiedebergs Arch Pharmacol. 2021 Apr;394(4):819-828. doi: 10.1007/s00210-020-02009-7. Epub 2020 Nov 2. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33136176
Cited by
-
Real-world evidence of fremanezumab for treating migraine in Japan: a retrospective study.BMC Neurol. 2023 Nov 14;23(1):404. doi: 10.1186/s12883-023-03449-3. BMC Neurol. 2023. PMID: 37964188 Free PMC article.
References
LinkOut - more resources
Full Text Sources

